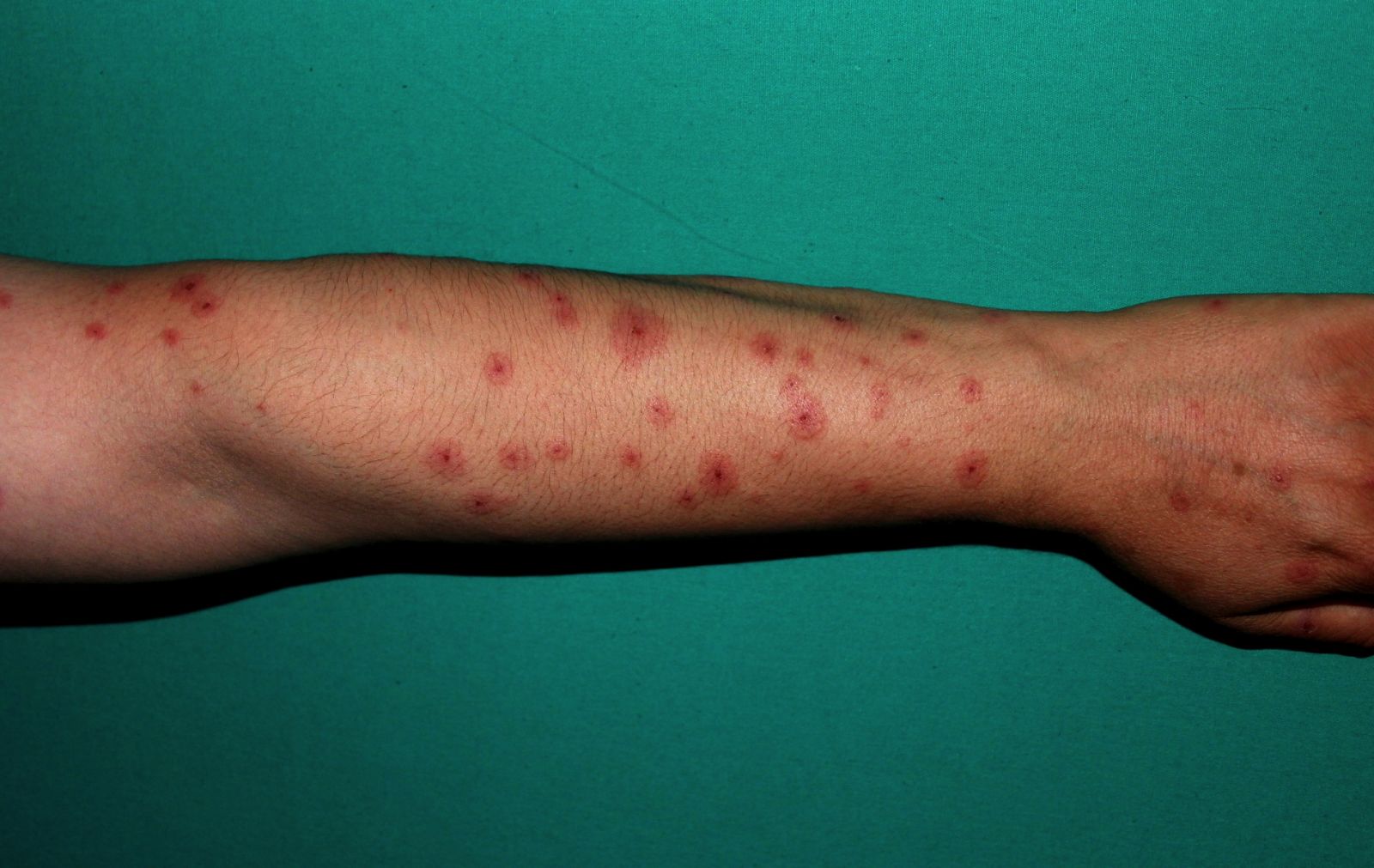
Antiepileptic Drugs (AEDs) linked to rare serious skin reactions.
Source: University of Rhode Island College of Pharmacy (Kingston) Nov 18, 2018 7 years, 3 months, 2 weeks, 2 days, 17 hours, 15 minutes ago
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic
drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in the journal: Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
Reference: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.