BREAKING COVID-19 NEWS: Yale Researchers Uncover Novel Insights Into SARS-CoV-2 Gene Expression Through Noncanonical Translation Factor eIF2A
Thailand Medical News Team Aug 15, 2023 2 years, 6 months, 1 week, 3 days, 4 hours, 30 minutes ago
COVID-19 News: The ongoing battle against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the global COVID-19 pandemic, has witnessed various scientific breakthroughs. Recent research from Yale University School of Medicine sheds light on a previously unexplored facet of SARS-CoV-2 gene expression, uncovering the role of a noncanonical translation factor known as eukaryotic translation initiation factor 2A (eIF2A) in enhancing programmed - 1 ribosomal frameshifting (-1 PRF). This discovery could have far-reaching implications for understanding the virus's replication process and potentially open doors to new antiviral strategies.
 Graphical Abstract
Programmed -1 Ribosomal Frameshifting: A Key Regulatory Mechanism
Graphical Abstract
Programmed -1 Ribosomal Frameshifting: A Key Regulatory Mechanism
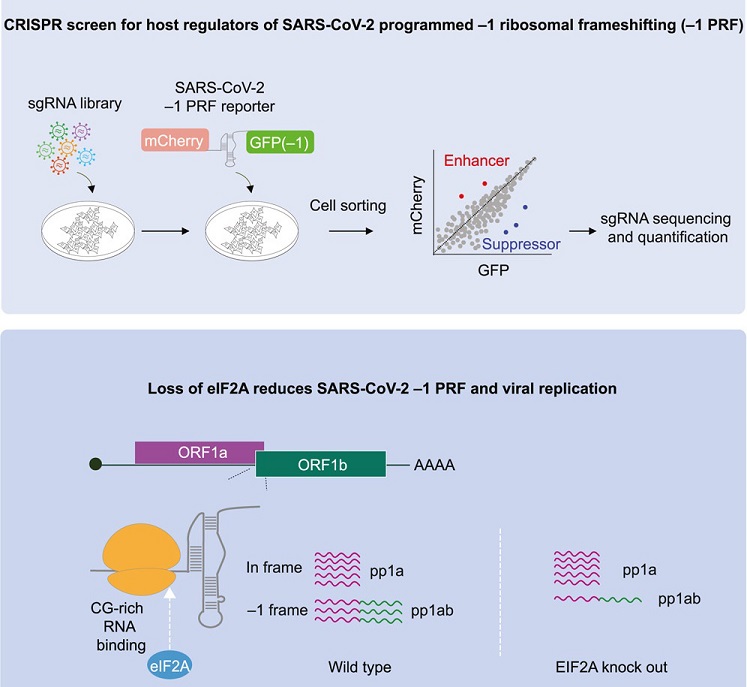
Many RNA viruses, including coronaviruses like SARS-CoV-2, utilize a mechanism called programmed −1 ribosomal frameshifting (−1 PRF) to regulate the translation of their polycistronic viral RNAs. This unique process involves a deliberate shift in the reading frame during translation, which allows the virus to synthesize critical proteins necessary for its replication. In the case of SARS-CoV-2, this mechanism is crucial for producing nonstructural proteins (NSPs) that play a pivotal role in viral replication.
CRISPR-Cas9 Screens Unveil Host Factors Modulating −1 PRF
The researchers at Yale University School of Medicine employed cutting-edge genome-wide CRISPR-Cas9 knockout screens to identify host factors that influence −1 PRF in SARS-CoV-2. These screens revealed several host factors that either suppress or enhance −1 PRF. Among them, eIF2A stood out as a central player in enhancing −1 PRF independently of its previously known role in translation initiation.
eIF2A's Dual Role: From Initiation to Enhanced −1 PRF
Eukaryotic translation initiation factor 2A (eIF2A) has long been recognized as a key player in translation initiation, facilitating the binding of initiator methionyl-tRNA to the ribosome. However, this study reveals a novel role for eIF2A in the context of SARS-CoV-2 gene expression. Contrary to its function during initiation, eIF2A was found to directly enhance −1 PRF, promoting the programmed frame shift that facilitates the translation of essential viral proteins.
Reduced SARS-CoV-2 Replication Upon Loss of eIF2A
The study team also investigated the consequences of eIF2A's involvement in −1 PRF. They found that the loss of eIF2A led to a reduction in SARS-CoV-2 replication within cells. This underscores the importance of efficient −1 PRF in driving t
he expression of viral proteins critical for viral replication. This discovery is the first and has never been covered in any past studies nor in any
COVID-19 News reports.
Binding Preferences: eIF2A's Selective Interaction with CG-Rich RNA Motifs
Delving further into eIF2A's behavior, the researchers discovered its preferential binding to RNA motifs rich in cytosine-guanine (CG) pairs. This binding pattern extended to the 18S ribosomal RNA (rRNA) region near the interaction points between the SARS-CoV-2 frameshift-stimulatory element (FSE) and the ribosome. This revelation offers insights into the potential mechanisms underlying eIF2A's effect on enhancing −1 PRF, as it suggests specific interactions between eIF2A, the ribosome, and the viral RNA pseudoknot.
Implications and Future Directions
This groundbreaking study not only expands our understanding of SARS-CoV-2 gene expression but also highlights the intricate ways in which host factors can modulate viral replication. The identification of eIF2A as a promoter of −1 PRF offers new avenues for antiviral research. By targeting eIF2A's role in enhancing −1 PRF, scientists could potentially hinder the virus's ability to replicate effectively, opening doors to innovative therapeutic strategies.
Furthermore, this study underscores the dynamic interplay between viruses and host cellular machinery. Viruses like SARS-CoV-2 have evolved to manipulate host factors to their advantage, and unraveling these interactions could lead to new insights into host-virus relationships that extend beyond the current pandemic.
In conclusion, the Yale University School of Medicine's research adds a significant piece to the intricate puzzle of SARS-CoV-2 biology. By shedding light on eIF2A's dual role and its impact on viral gene expression, this study could have far-reaching implications for understanding and combatting the virus that has shaped the global landscape in unprecedented ways.
The study findings were published in the peer reviewed journal: Cell Reports.
https://www.sciencedirect.com/science/article/pii/S2211124723009981
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
