COVID-19 News: University Of Texas Scientists Uncover Multiple Roles Of NSP6 In SARS-CoV-2 Pathogenesis
COVID-19 News - NSP6 Apr 03, 2023 2 years, 10 months, 2 weeks, 6 days, 15 hours, 56 minutes ago
COVID-19 News: Since its emergence in late 2019, the SARS-CoV-2 virus has continued to evolve and adapt. As the virus responsible for COVID-19, researchers have extensively investigated its replication and pathogenesis to develop vaccines and treatments. The viral spike protein has been the main focus due to its role in infection and transmission. However, other viral proteins have been less studied.
 SARS-CoV-2 NSP6
SARS-CoV-2 NSP6
Recent research has identified nonstructural protein 6 (nsp6) as a key player in SARS-CoV-2 replication, bridging this knowledge gap.
Interestingly, a past
COVID-19 News coverage showed that NSP6 proteins could even induce morphological and structural defects in the heart!
https://www.thailandmedical.news/news/breaking-university-of-maryland-study-shockingly-finds-that-sars-cov-2-nsp6-proteins-can-induce-morphological-and-functional-defects-in-the-heart
Nsp6 is involved in forming replication organelles, counteracting type I interferon (IFN-I) responses, and activating NLRP3 inflammasomes…all significant factors that contribute to COVID-19 severity.
In this new study, the researchers from University of Texas Medical Branch, Galveston, Texas-USA presents the latest findings on nsp6's multiple roles in SARS-CoV-2 replication and pathogenesis. It covers the structural model and function of the SARS-CoV-2 nsp6 protein, highlighting its importance as a membrane protein for replication organelle formation, its antagonism of type-I interferon responses and NLRP3 inflammasome activation, and the specific nsp6 mutations in SARS-CoV-2 variants that modulate viral pathogenesis.
Although all nonstructural proteins play critical roles in replication, the molecular structure of nsp6 remains unsolved. However, recent studies suggest that nsp6 contributes to SARS-CoV-2 replication through various mechanisms. Three primary functions have been reported for nsp6: (i) forming replication organelles through dimerization, (ii) disrupting IFN-I signaling pathways to counteract host innate immune response, and (iii) activating NLRP3 inflammasomes by inhibiting lysosome acidification.
Nsp6 Structure
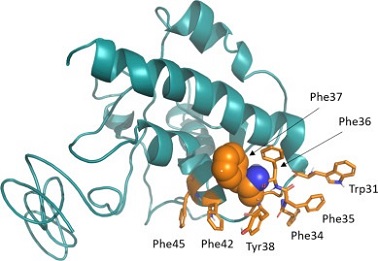
Coronavirus nsp6 is a 34 kDa transmembrane protein found in the ER membrane and perinuclear space. SARS-CoV-2 nsp6 shares 87% identity with SARS-CoV nsp6. Common SARS-CoV-2 variants have a three amino-acid deletion (ΔSGF). Predictive software offers contradictory results for nsp6's structure. AlphaFold predicts 8 transmembrane domains, while experimental evidence supports 7 transmembrane domains. An atomic structure of nsp6 is needed to validate these models.
Double Membrane Vesicle Formation
SARS-CoV-2 nsp3, nsp4, and nsp6 proteins modify ER membranes to form double membrane vesicles (DMVs), providing a protective environment for viral RNA replication. Nsp6 is involved in DMV formatio
n and restricts access to the compartments. Lipid droplets (LDs) are required for SARS-CoV-2 replication and replenish lipids in DMV structures. Co-expression of nsp3/nsp4/nsp6 produces tighter clusters of DMVs with smaller diameters compared to those produced by nsp3/nsp4 expression without nsp6. Enhanced zippering activity of nsp6(ΔSGF) may contribute to immune evasion in variants containing ΔSGF.
Modulation of Autophagy
Autophagy is crucial in managing cellular waste and destroying intracellular infectious material. SARS-CoV-2 nsp6 inhibits the formation of hybrid pre-autophagosomal structures (HyPAS), resulting in smaller autophagosomes that degrade viral components less efficiently. Late in the infection, nsp6 reduces autophagy and prevents the fusion of compartments containing viral components with lysosomes.
NLRP3 Inflammasome Activation and Pyroptosis
Nsp6 inhibits the lysosome-autophagy system, leading to the accumulation of non-digestive autophagosomes and activation of the NLRP3 inflammasome. This triggers an inflammatory form of apoptosis called pyroptosis, which may contribute to severe COVID-19 symptoms.
Interaction with Host Factors and Potential Drug Targets Studies have identified high-confidence nsp6 interactors, including Sigma-1 receptor (SIGMAR1) and components of the vacuolar ATPase. These interactors are potential drug targets, and compounds like haloperidol may be effective at reducing SARS-CoV-2 infection in vitro.
Nsp6 Antagonism of IFN-I Pathways
Nsp6 inhibits IFN-I production and signaling pathways, thereby suppressing the host's immune response. Variants of SARS-CoV-2 with specific nsp6 mutations have shown enhanced resistance to IFN treatment.
Activation of NF-κB Expression
Nsp6 interacts with host factors like transforming growth factor β-activated kinase 1 (TAK1), leading to the activation of the NF-κB pathway. This activation results in increased mRNA expression of proinflammatory cytokines such as IL-8 and IP-10, which are associated with severe COVID-19.
NSP6 Mutations in Vivo
It was demonstrated that nsp6 mutations not only enhance the suppression of IFN-I signaling pathways but also result in greater viral RNA production in the lungs of mice infected with a mutant WA1 SARS-CoV-2 containing the ΔSGF nsp6 deletion (ΔSGF-WA1) compared to the parental WA1 virus. Mice infected with ΔSGF-WA1 experienced weight loss sooner, and their illness lasted longer than those infected with the WA1 virus. The survival rate for ΔSGF-WA1-infected mice was also lower, at 50% compared to 75% for WA1-infected mice.
Surprisingly, lung tissue histopathology from both groups of mice was similar, indicating that ΔSGF-WA1 does not cause a more severe cytopathic effect in the lungs. Instead, an analysis of the host response revealed that cytokine pathways and pathogen-induced cytokine storm pathways were initially downregulated but later significantly upregulated in both mouse lung tissues and infected primary human airway epithelial cells. This suggests that ΔSGF-WA1 efficiently represses early immune responses to promote viral replication, which leads to an overcompensating immune system and cytokine storm, likely resulting in organ failure and death. This pattern is consistent with COVID-19 in humans, where IFN-I response is delayed during early infection, followed by a proinflammatory response in the later stages of the disease.
A study found that parental SARS-CoV-2 containing the BA.1 S gene combined with BA.1 nsp6 (ΔLSG + I189V) was significantly attenuated in recombinant ACE2/TMPRSS2/Caco-2 cells and K18-hACE2 mice, resembling the attenuated phenotype of full-length Omicron BA.1. Interestingly, mice infected with the recombinant BA.1 S virus had a survival rate of 20%, while 71% of mice infected with the BA.1 S/nsp6 virus and 100% of full-length BA.1-infected mice survived. This supports previous findings that mutations in the 5′-UTR-nsp12 region attenuate SARS-CoV-2 replication in K18-hACE2 mice, while BA.1 S mutations increase virulence. However, it contrasts with the current study finding that mice infected with ΔSGF-WA1 had increased mortality.
The discrepancy is unclear since ΔLSG, ΔLSG+I189V, and ΔSGF nsp6 all displayed enhanced suppression of IFN-I signaling in vitro. It has been suggested that BA.1 S mutations may alter viral tropism, while nsp6 mutations could serve as an adaptation to a changed tissue environment. Whether BA.1 S and nsp6 work together is uncertain. The contrasting outcomes of ΔSGF-WA1 and BA.1 S/nsp6 infections may indicate an epistatic interaction between the S and nsp6 genes.
C
onclusions
The COVID-19 pandemic has had a devastating global impact, causing significant social and economic disruptions. The swift development and approval of vaccines have altered the pandemic's trajectory and saved countless lives. However, future coronavirus variants and other coronaviruses with pandemic potential remain a threat. Therefore, it is crucial to continue studying coronaviruses, particularly the roles of nonstructural and accessory proteins. While several functions of the SARS-CoV-2 nsp6 protein have been identified, many questions persist. Determining the atomic structure of nsp6 will offer essential insights into its molecular mechanisms, such as how ΔSGF and ΔLSG mutations impact protein-protein interactions or how L37F hinders them. This structural information could also enhance the understanding of nsp6-mediated DMV formation and aid in designing inhibitors of its function. Identifying specific interaction partners is critical for comprehending nsp6's antagonism of IFN-I pathways and other functions.
The study findings were published in the peer reviewed journal: Antiviral Research
https://www.sciencedirect.com/science/article/pii/S0166354223000682
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
