Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 11, 2025 4 months, 2 days, 22 hours, 28 minutes ago
Pharma News: A Major Step in Heart Failure Management
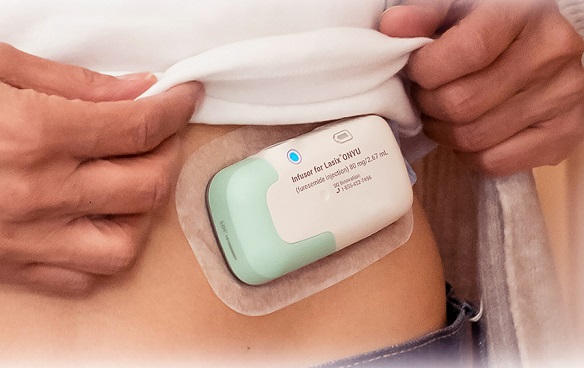
Millions of Americans living with heart failure may soon find it easier to manage their condition from the comfort of home. The U.S. Food and Drug Administration (FDA) has approved Lasix ONYU, a new at-home version of the widely used diuretic furosemide injection, developed by SQ Innovation, Inc. This novel drug-device combination allows patients to receive subcutaneous (under-the-skin) infusions at home instead of undergoing intravenous (IV) therapy in a hospital setting.
 United States FDA Approves Home Use Version of Lasix for Heart Failure
United States FDA Approves Home Use Version of Lasix for Heart Failure
In this
Pharma News report, it was highlighted that Lasix ONYU’s approval could dramatically change how fluid overload and edema in chronic heart failure are treated. According to Dr. Pieter Muntendam, President and CEO of SQ Innovation, this marks a breakthrough in patient-centered care. “Lasix ONYU has the potential to be transformative in the care of patients experiencing worsening heart failure due to fluid overload,” he stated. He added that home-based treatment could benefit patients, health systems, and insurers alike.
An Expanding Public Health Concern
Currently, more than 6.7 million Americans live with heart failure, a figure projected to rise to 8.7 million by 2030. The disease is one of the leading causes of hospitalization for older adults, resulting in around 1.2 million hospital admissions each year. The conventional treatment often involves IV Lasix administered in hospitals, which can be resource-intensive and costly. Lasix ONYU seeks to relieve that burden by offering a safe, effective, and cost-efficient alternative for selected patients.
How the At-Home System Works
Lasix ONYU’s design consists of two key components — a reusable unit lasting for 48 treatments and a single-use cartridge discarded after each session. The dual-part system not only lowers production costs but also enhances patient convenience. Clinical trials revealed that the drug demonstrated 112% bioavailability compared to the IV version, meaning it was even more efficiently absorbed. The results also showed similar or better outcomes in urine output (115%) and sodium loss (117%), confirming that home use is as effective as hospital-based care.
Expert Endorsements and Future Impact
Dr. Javed Butler, Professor of Medicine at the University of Mississippi and President of the Baylor Scott & White Research Institute in Dallas, emphasized the growing challenge in managing the heart failure epidemic. “Heart failure is the most common serious medical condition in the U.S. and affects about 1 in 4 Americans during their lifetime. We already lack adequate resources to manage the 6.7 million cases currently diagnosed,” he noted. Butler added that allowing home treatment could alleviate strain on hospitals, clinicians, and healthcare funding.
Nursing professionals also welcomed the innovation. S. Craig Thomas, past president of the American Association of
Heart Failure Nurses, explained that IV diuretics have been the standard for managing fluid retention for more than 50 years. “The availability of accessible, affordable, and novel options that do not require the presence of a health care professional allows for transformative new clinical care delivery,” he said. With Lasix ONYU, patients who typically require several days of hospital-based IV therapy could instead receive treatment at home, monitored remotely by healthcare teams.
Wider Access and Broader Implications
The newly approved therapy will be distributed through major pharmaceutical channels within this quarter, making it accessible at selected hospitals and pharmacies nationwide. SQ Innovation plans to roll out the product with leading health systems before the year’s end, signaling a potential shift in chronic disease management models.
This development represents a crucial milestone for both patients and the healthcare system. The ability to safely administer Lasix outside the hospital not only improves patient comfort and adherence but also reduces hospital congestion and healthcare costs. In the long term, innovations like Lasix ONYU could pave the way for broader adoption of home-based treatments for other chronic illnesses, redefining modern medicine’s approach to long-term care.
For the latest
Pharma News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/us-fda-approves-new-antibacterial-drug-fetroja-(cefiderocol)-to-treat-complicated-urinary-tract-infections
https://www.thailandmedical.news/news/long-term,-daily-low-dose-aspirin-intake-does-not-prevent-incidence-of-stroke,-instead-increases-risk-of-intracerebral-bleeding-in-older-adults-by-38-
https://www.thailandmedical.news/news/us-fda-approves-new-antibacterial-drug-fetroja-(cefiderocol)-to-treat-complicated-urinary-tract-infections
