SARS-CoV-2 Upregulates CD55 Complement Regulator And Prolongs Its Activation Causing An Immune Dysfunction That Contributes To Disease Severity In Some
Source: COVID-19 Immunology Oct 08, 2022 3 years, 4 months, 2 weeks, 2 days, 19 hours, 49 minutes ago
COVID-19 Immunology: A new study by Greek researchers from Kapodistrian University of Athens, Evangelismos General Hospital-Greece and the Institute for Bioinnovation-Greece has found that the upregulation of CD55 complement regulator in distinct PBMC subpopulations of COVID-19 patients is associated with suppression of interferon responses.
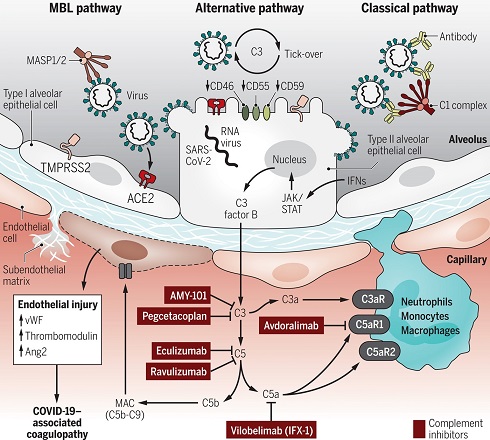
The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and attack the pathogen's cell membrane. It is part of the innate immune system, which is not adaptable and does not change during an individual's lifetime.
The complement system basically acts as an immune surveillance mechanism constantly in search of pathogens. Upon recognition of pathogens such as bacteria or viruses its activation results in a cascade of reactions ultimately leading to the lysis and killing of the identified pathogen
The complement system consists of a number of small proteins that are synthesized by the liver, and circulate in the blood as inactive precursors. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this complement activation or complement fixation cascade is stimulation of phagocytes to clear foreign and damaged material, inflammation to attract additional phagocytes, and activation of the cell-killing membrane attack complex. About 50 proteins and protein fragments make up the complement system, including serum proteins, and cell membrane receptors. They account for about 10% of the globulin fraction of blood serum.
Activation of the complement cascade may be regarded as a protective mechanism ultimately leading to the elimination of virus particles and disease progression.
However, uncontrolled or prolonged complement activation may have a negative effect contributing to disease pathogenesis.
Complement activation has been verified in COVID-19 patients by both increased serum levels of complement factors C3a and C5b-9 and increased complement deposition at the tissue levels. Complement regulatory proteins (CRPs) CD55, CD46, CD59 and CR1 act to control complement overactivation and eliminate complement deposition and cell lysis.
The
COVID-19 Immunology study team aimed to investigate the expression of CRPs in COVID-19 in order to identify potential dysregulated expression patterns of CRPs and address whether these may contribute to disease pathogenesis.
Single cell RNA-sequencing (scRNA-seq) analysis performed on isolated PBMCs revealed an increase of CD55 expression in severe and critical COVID-19 patients compared to healthy controls.
This increase was also detected upon integrated sub-clustering analysis of the monocyte, T cell and B cell populations. Flow cytometric analysis verified the distinct pattern of upregulated CD55 expression in monocyte and T cell sub populations of severe COVID-19 patients.
Importantly, this upregulation was associated with decreased expre
ssion of interferon stimulated genes (ISGs) in patients with severe COVID-19 suggesting a potential suppressor effect of CD55 on interferon responses.
The study findings identify a COVID-19 specific CD55 expression pattern in PBMC subpopulations that coincides with reduced interferon responses thus indicating that the complement regulator CD55 may contribute to COVID-19 pathogenesis.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2022.10.07.510750v1
The study findings show that a dysregulated expression pattern of CRPs may be present in COVID-19 contributing to complement overactivation.
The study involving sc-RNAseq analysis of PBMCs isolated from COVID-19 patients identified a distinct COVID-19 specific expression pattern for CD55 in severely and critically ill patients.
The upregulation of CD55 expression observed could be explained as a putative protective mechanism of blood cell subpopulations from uncontrolled complement activation.
The upregulation of CD55 may therefore act to eliminate further activation and deposition of complement in this cellular population
The study team also observed an increase in the expression of CD59, which acts on the final step of the cascade inhibiting C5b-9 formation and deposition and therefore complement mediated cell lysis, in monocytes and granulocytes of patients with severe and critical COVID-19 with no upregulation in T cells or B lymphocytes.
The increase of CD55 observed in the specific cellular populations of COVID-19 patients with severe and critical disease may be taking place as an additional complement activation halting mechanism in order to achieve protection of these cell populations.
The researchers also observed an upregulation of CD46 in monocytes as well as in the T and B lymphocytes by scRNA-seq analysis. Augmented CD46 expression levels in monocytes have been reported in patients with viral infections with a concomitant downregulation of CD46 expression in T cells. Although not verified at the protein level the T cell CD46 upregulation may also present a COVID-19 specific expression for CD46.
The study findings indicate that the enhanced CD55 and CD46 expression levels observed in PBMCs during COVID-19 may signify an effort to activate the halting machinery of complement activation in order to protect PBMCs which are the first line of defense during the infection.
Interestingly, the study team also observed a reduction of CD55 expression in the monocyte population of the critical COVID-19 group of patients in comparison to the severe group. This decrease may contribute to the sustained complement deposition reported in the monocyte population of convalescent individuals.
The distinct feature of CD55 upregulation confirmed in monocytes and T and B lymphocytes of COVID-19 severe and critical patients was associated with reduced type I interferon responses.
Type I interferon responses are key mediators of the antiviral defense and involve IFN-a and IFN-β.
A suppression of type I interferon responses has been well documented in patients with severe and critical COVID-19.
The study findings also confirmed the profile of reduced type I interferon responses in the severe and critical COVID-19 groups of patients following a marked increase in the mild COVID19 group by analysis of the expression of nine ISGs in both total PBMCs as well as in the monocyte, and T and B lymphocyte subpopulations.
Interestingly, the reduction of ISGs expression in the severe and critical COVID-19 groups of patients coincided with the upregulation of CD55 expression which was also confirmed at the protein level by FACS analysis in each PBMC subpopulation, thus strengthening the validity of the single-cell analysis.
The association of CD55 upregulation with reduced ISGs expression identified in monocytes as well as in T and B lymphocytes points to a possible involvement of CD55 in the repression of interferon responses signaling displayed in these cellular populations.
CD55 has also been linked to the suppression of adaptive immune responses in-vivo.
In one study involving CD55 knock-out mice, CD4+ T-cells were reported to excessively produce IFN-γ and IL-2 in response to active immunization.
https://pubmed.ncbi.nlm.nih.gov/15710649/
CD55 has also been linked to the suppression of NK cell responses.
https://pubmed.ncbi.nlm.nih.gov/1381394/
The association of increased CD55 expression in monocytes T cells and B cells of patients with severe and critical COVID-19 with a reduction of ISGs expression identified in the study is therefore a strong indication of a possible contribution of CD55 in the suppression of type I interferon responses in COVID-19 patients.
The study team concluded, “In summary, in the present scRNA-seq study, we identified a COVID-19 specific expression pattern for complement regulatory protein CD55 characterized by upregulation in monocyte, CD4+ and CD8+ T cells as well as B cell lineage clusters. Increased expression of CD55 in these subpopulations was associated with decreased type I interferon responses, indicating a potential role of CD55 in COVID-19 severe and acute pathogenesis by promoting suppression of interferon responses. The present study further strengthens the importance of complement activation in COVID-19 and introduces novel molecules of the complement cascade as COVID-19 mediators. Given the diverse array of complement targeted therapeutic strategies our findings may point towards novel therapies in the fight against COVID-19.”
For the latest on
COVID-19 Immunology, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/stanford-study-shows-that-the-sars-cov-2-coronavirus-is-able-to-evade-cytotoxic-responses-of-natural-killer-cells-and-cause-immune-dysfunction
https://www.thailandmedical.news/news/yale-study-discovers-that-sars-cov-2-proteins-orf7a-and-orf3a-downregulates-mhc-i-expression-aiding-in-immune-evasion-and-immune-dysfunction
https://www.thailandmedical.news/news/even-mild-and-moderate-sars-cov-2-infections-causes-transcriptional-reprogramming-of-the-host-s-cd14-monocytes-resulting-in-dysfunctional-monocytes
https://www.thailandmedical.news/news/german-study-shockingly-discovers-that-sars-cov-2-caused-complement-activation-induces-excessive-cd16-t-cells-with-increased-cytotoxic-functions
https://www.thailandmedical.news/news/study-shows-sars-cov-2-reduces-the-numbers-and-functional-competence-of-dendritic-cells-in-circulation-and-affect-responses-to-secondary-infections
https://www.thailandmedical.news/news/breaking-macrophage-induced-protein-spp1-identified-as-driving-multiple-pathogenic-inflammatory-responses-in-severe-covid-19-and-long-covid
https://www.thailandmedical.news/news/neutrophil-dysregulation-observed-in-severe-covid--iga-dominant-responses-drive-neutrophil-effector-functions-causing-disease-severity-and-mortality
https://www.thailandmedical.news/news/covid-19-induces-expression-of-unique-autoantibodies-irrespective-of-symptomatic-or-asymptomatic-infections-males-at-risk-for-autoimmune-issues
https://www.thailandmedical.news/news/covid-19-immunology-netherlands-study-finds-that-sars-cov-2-infection-activates-dendritic-cells-via-cytosolic-receptors-rather-than-extracellular-tlrs
https://www.thailandmedical.news/news/uk-study-shows-that-sars-cov-2-spike-s1-glycoprotein-is-a-tlr4-agonist,-upregulates-ace2-and-induces-pro-inflammatory-m1-macrophage-polarization
https://www.thailandmedical.news/news/university-of-california-study-shows-that-exhausted-t-cells-are-linked-to-increased-risk-of-covid-19-death
https://www.thailandmedical.news/news/covid-19-immunology:-sars-cov-2-modulates-dendritic-cell-maturation-and-suppresses-the-signaling-cascade-necessary-for-t-cell-activation
https://www.thailandmedical.news/news/covid-19-immunology-yet-another-study-shows-that-sars-cov-2-suppresses-human-host-innate-immunity
https://www.thailandmedical.news/news/covid-19-immunology-study-reveals-that-sars-cov-2-causes-cd4-t-cells-and-cd25-hyperactivation-while-repressing-foxp3-genes-in-severe-covid-19
https://www.thailandmedical.news/news/covid-19-immunology-study-shows-that-sars-cov-2-viral-protein-orf3a-activates-nlrp3-inflammasome-causing-severe-inflammatory-responses
https://www.thailandmedical.news/news/covid-19-immunology-swedish-study-shows-natural-killer-cell-immunotypes-linked-to-covid-19-disease-severity
https://www.thailandmedical.news/news/breaking-covid-19-immunology-could-sars-cov-2-be-depleting,-altering-or-even-destroying-cd8-cells-new-australian-study-suspects-so
https://www.thailandmedical.news/news/breaking-covid-19-immunology-stanford-university-study-shows-that-sars-cov-2-impairs-and-dysregulates-the-immune-system
https://www.thailandmedical.news/news/breaking!-covid-19-immunology-study-shows-high-levels-of-t-cells-with-significantly-altered-function-and-phenotype-in-severe-covid-19-patients
