Glaucoma News: University Of Wisconsin Study Finds That Integrins Play A Role In In The Development Of Fibrosis In The Trabecular Meshwork
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 28, 2023 2 years, 3 months, 4 weeks, 1 hour, 3 minutes ago
Glaucoma News: Primary open-angle glaucoma (POAG) is a progressive and chronic eye disease characterized by the development of fibrosis in the trabecular meshwork (TM). The extracellular matrix (ECM) within the TM undergoes extensive remodeling and stiffening, mimicking fibrotic changes. This fibrosis in the TM is associated with myofibroblast activation and increased cell contractility, contributing to tissue stiffening. In this
Glaucoma News report, we will explore the role of integrins in the development of fibrosis within the TM, shedding light on their involvement in the pathogenesis of POAG.
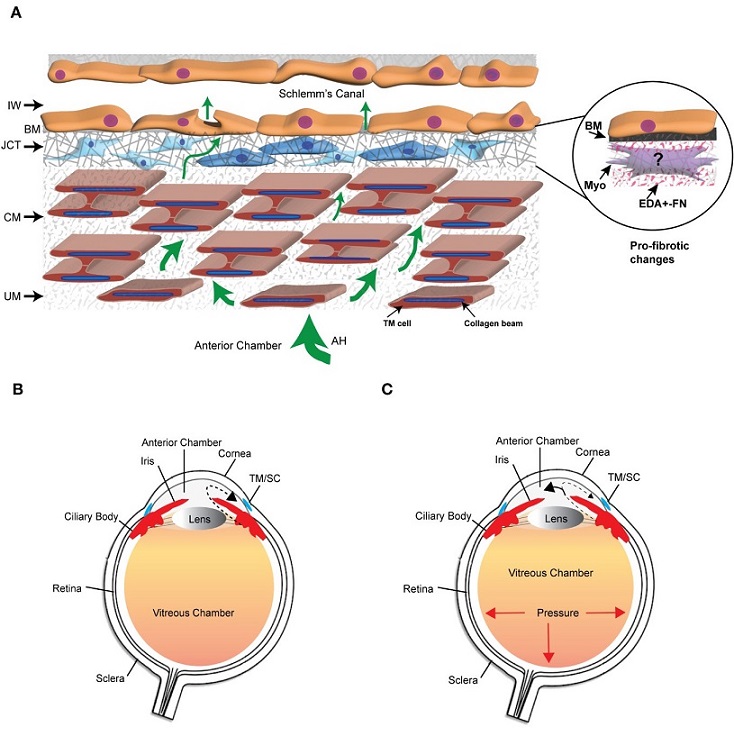 The trabecular meshwork/Schlemm’s canal (TM/SC) outflow pathway. (A) Aqueous humor (AH, green arrows) exits the anterior chamber through the uveoscleral meshwork (UM), corneoscleral meshwork (CM) and the juxtacanalicular tissue (JCT). It then crosses the basement membrane (BM) underlying the inner wall (IW) of Schlemm’s Canal to exit either paracellularly or transcellularly into the lumen of SC. The light and dark blue cells in the JCT indicate that the JCT consists of cells showing both fibroblastic and smooth muscle-like properties, respectively. The beams are connected to each other by cytoplasmic extensions between the TM cells surrounding the beams. The insert in the circle shows the profibrotic changes associated with POAG that may include the transition of TM cells in the JCT into myofibroblasts, the expression of the EDA+ isoform of fibronectin (FN), and the increased production of proteins in the BM of the IW wall. (B) Diagram of the whole eye showing the normal movement of aqueous humor (dashed arrow) from the ciliary body past the lens and iris into the anterior chamber and out through the TM/SC. (C) Diagram of the whole eye showing that profibrotic changes in the TM/SC shown in (A) would lead to a restriction in the movement of aqueous humor through the TM/SC (smaller arrow head) and an accumulation of more aqueous humor in the anterior chamber (larger arrowhead). This would result in increased pressure throughout the eye including in the vitreous chamber.
The Trabecular Meshwork and Fibrosis
The trabecular meshwork/Schlemm’s canal (TM/SC) outflow pathway. (A) Aqueous humor (AH, green arrows) exits the anterior chamber through the uveoscleral meshwork (UM), corneoscleral meshwork (CM) and the juxtacanalicular tissue (JCT). It then crosses the basement membrane (BM) underlying the inner wall (IW) of Schlemm’s Canal to exit either paracellularly or transcellularly into the lumen of SC. The light and dark blue cells in the JCT indicate that the JCT consists of cells showing both fibroblastic and smooth muscle-like properties, respectively. The beams are connected to each other by cytoplasmic extensions between the TM cells surrounding the beams. The insert in the circle shows the profibrotic changes associated with POAG that may include the transition of TM cells in the JCT into myofibroblasts, the expression of the EDA+ isoform of fibronectin (FN), and the increased production of proteins in the BM of the IW wall. (B) Diagram of the whole eye showing the normal movement of aqueous humor (dashed arrow) from the ciliary body past the lens and iris into the anterior chamber and out through the TM/SC. (C) Diagram of the whole eye showing that profibrotic changes in the TM/SC shown in (A) would lead to a restriction in the movement of aqueous humor through the TM/SC (smaller arrow head) and an accumulation of more aqueous humor in the anterior chamber (larger arrowhead). This would result in increased pressure throughout the eye including in the vitreous chamber.
The Trabecular Meshwork and Fibrosis
The TM, located in the anterior segment of the human eye, consists of various extracellular matrix (ECM) proteins, including different types of collagens (I, III, IV, V, VI), fibronectin, laminin, and hyaluronan. Elastic fibers composed of fibrillin and elastin, as well as matricellular proteins, are also present within the TM. This complex network of ECM components is distributed throughout the layers of the TM, with the uveal and corneoscleral meshworks forming trabecular lamellae and the juxtacanalicular tissue (JCT) containing a combination of fibroblastic and smooth muscle-like cells within an ECM rich in collagens, elastin fibers, fibronectin, hyaluronan, and proteoglycans.
The TM and the inner wall of Schlemm's Canal (SC) are crucial for regulating the outflow of aqueous humor and intraocular pressure (IOP). However, excessive and prolonged remodeling of the ECM within the JCT and the inner wall of SC leads to a restriction in aqueous humor outflow, resulting in elevated IOP. This elevation in IOP is a key factor in the pathogenesis of POAG. The transition into a pro-fibrotic state wi
thin the TM can be triggered by age-related ECM remodeling, elevated levels of transforming growth factor-beta 2 (TGFβ2) in aqueous humor, or other signaling cascades, such as Wnt/β-catenin, Notch, and inflammatory cytokines. This process, known as endothelial-to-mesenchymal transition (EndoMT), can result in the transformation of quiescent TM cells into myofibroblast-like cells, which further promotes fibrosis and tissue stiffening.
Integrins in the Trabecular Meshwork
Integrins, a family of transmembrane receptors, are instrumental in the regulation of various signaling pathways involved in fibrosis, including TGFβ signaling, fibronectin matrix formation, myofibroblast formation, and activation of Rho GTPases. Integrins can mediate both cell attachment to the ECM and transduce extracellular signals into intracellular responses.
There are 18 alpha (α) and 8 beta (β) subunits in humans, forming 24 unique integrin receptors with tissue-specific properties and ligand specificity. Integrins are broadly distributed in the TM, with key integrins identified in the JCT and SC cells, including αvβ5 and α9β1. These integrins play a pivotal role in mediating fibrotic processes in the TM.
Integrins and Rho GTPases in the TM
The Rho GTPase pathway, involving RhoA, Rac, and Cdc42, is essential for regulating the contractile and phagocytic properties of the TM and myofibroblast differentiation. Integrins control the activation and localization of Rho GTPases, thereby affecting contractility and ECM deposition. α5β1 integrin is a critical player in promoting fibronectin fibril formation, a key process in fibrosis. This integrin triggers the reorganization of actin and α-smooth muscle actin (α-SMA) into crosslinked actin networks (CLANs) through the activation of Rac1, leading to altered TM contractility and mechanical properties.
On the other hand, αvβ3 integrin enhances fibronectin fibril formation and the deposition of fibronectin fibrils. Unlike α5β1 integrin, αvβ3 integrin employs a RhoA/ROCK-independent process, suggesting that it may activate alternative pathways, such as GEF-H1/mDia or Rac1, to induce contractile forces in TM cells. This dual mechanism of integrin-mediated contractility, involving both α5β1 and αvβ3 integrins, may be responsible for regulating fibril formation in the TM and could be targeted for treating POAG.
Role of α5β1 and αvβ3 Integrins in Fibronectin Matrix Formation
Integrins are pivotal in the formation of fibronectin fibrils, which are essential for maintaining the ECM architecture during fibrosis. Fibronectin, a glycoprotein with various domains, interacts with multiple binding partners within the ECM. Fibronectin fibrillogenesis is a crucial process in fibrosis, and its disruption has shown promise in preventing fibrosis in various disease models. In the TM, α5β1 integrin plays a major role in promoting fibronectin fibril formation by inducing a conformational change in fibronectin, exposing specific binding sites necessary for fibril assembly.
The assembly of fibronectin into fibrils is regulated by integrins through the control of actomyosin network contractile forces. This process usually involves RhoA activation, which occurs when α5β1 integrin engages fibronectin. In contrast, αvβ3 integrin activation enhances the assembly of fibronectin into fibrils, potentially through an alternative mechanism that does not involve RhoA/ROCK. Integrins, therefore, play a critical role in modulating fibronectin fibrillogenesis in the TM.
Integrins in Contractility and Myofibroblast Differentiation
Myofibroblasts, characterized by the expression of α-SMA and the presence of contractile stress fibers, play a significant role in fibrosis. These cells are found in the TM of young individuals and decrease with age, but their levels increase following corticosteroid treatments. α-SMA-positive stress fibers, driven by contractile properties, contribute to the pathogenesis of fibrosis. Integrins are involved in the formation and maintenance of these stress fibers, and their contractile force generation contributes to myofibroblast transformation.
The transformation of cells into myofibroblasts involves two stages. First, the expression of EDA+ fibronectin initiates the process, which is induced by TGFβ1. Integrins such as α4β1, α4β7, or α9β1 may be involved in this transformation. The second stage involves the formation of α-SMA-containing stress fibers, which increase the release of TGFβ2 from the ECM and contribute to ECM rigidity. Integrins are central in both stages of myofibroblast maturation.
Integrins and TGFβ Signaling
TGFβ signaling is a major driver of myofibroblast differentiation and fibrosis. TGFβ2, in particular, is associated with POAG. Integrins may be involved in the activation and regulation of TGFβ2. Activation of αvβ3 integrin has been shown to increase the expression of TGFβ2 mRNA and protein in TM cells. This process may be modulated by calcineurin (CaN) and the transcription factor NFATc1 and is dependent on the proliferative state of TM cells.
Integrins can also regulate the association of the TGFβ receptors TGFβRI and TGFβRII, which are crucial for TGFβ signaling. αvβ3 integrin recruits TGFβRII to interact with TGFβRI in focal adhesions, promoting TGFβ signaling. Conversely, α2β1 integrin in focal adhesions negatively regulates TGFβRII phosphorylation, modulating TGFβ signaling. Therefore, integrins have the capacity to modulate TGFβ signaling, which is critical in fibrosis.
Conclusion
In summary, integrins are key players in various stages of fibrosis in the trabecular meshwork, from the deposition of ECM components to the modulation of TGFβ signaling and the contractility of myofibroblasts. Targeting integrins and their associated signaling pathways holds promise as a long-term antifibrotic strategy in chronic fibrotic diseases, aiming to restore the homeostasis of the TM and preserve its function. Disrupting fibronectin fibril formation and using recombinant integrin blocking antibodies are potential approaches to mitigate fibrosis in the TM. Understanding the intricate role of integrins in fibrosis is crucial for advancing the treatment of diseases like POAG and improving the quality of life for affected individuals.
The study findings were published in the peer reviewed journal: Frontiers in Ophthalmology.
https://www.frontiersin.org/articles/10.3389/fopht.2023.1274797/full
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
