University Of Colorado Study Uncovers Role Of Local Complement In Lung Endothelial Cell Activation During SARS-CoV-2 Infection
Thailand Medical News Team Aug 24, 2023 2 years, 5 months, 3 weeks, 4 hours, 27 minutes ago
COVID-19 News: The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed significant challenges to global healthcare systems. While the primary target of viral invasion is lung alveolar epithelial cells, emerging evidence suggests that endothelial dysfunction and inflammation also contribute to the vascular pathology associated with COVID-19. However, the exact mechanisms underlying the involvement of the vascular system and the role of local complement activation in this process remain unclear. In a groundbreaking study conducted at the University of Colorado Anschutz Medical Campus, researchers aimed to investigate whether lung endothelial cells and other resident vascular cells are susceptible to productive SARS-CoV-2 infection and how local complement activation contributes to endothelial dysfunction and inflammation in response to hypoxia and SARS-CoV-2-infected lung alveolar epithelial cells.
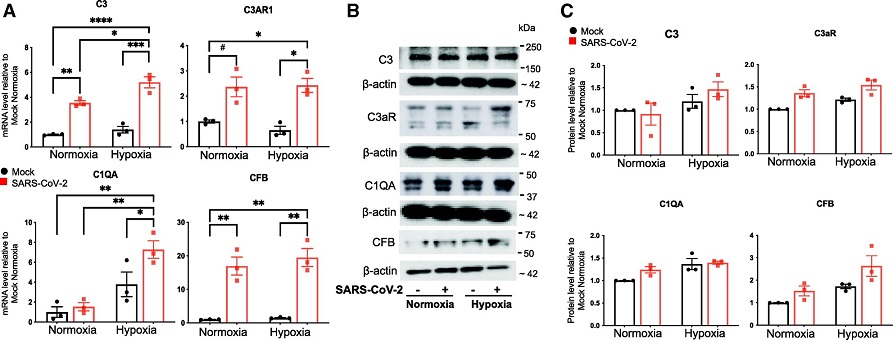 Effects of the conditioned medium of SARS-CoV-2–infected hACE2-A549 cells and hypoxia on the expression of complement proteins in HLMVECs. HLMVECs were preexposed to normoxic or hypoxic (3% O2) conditions for 24 hours, followed by the addition of conditioned medium from mock-infected or SARS-CoV-2–infected hACE2-A549 cells for 24 hours. (A) Total RNA was isolated, and mRNA concentrations of C3 (complement C3), C3AR1 (complement C3a receptor 1), C1QA (complement C1q A chain), and CFB (complement factor B) were determined using qRT-PCR analysis. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and #P = 0.0508 (borderline significant). (B) Total cell lysates were collected and analyzed for the expression of indicated complement components using western blot analysis, and the representative blots are presented. (C) Densitometry of the blots in B is presented (technical replicates).
Effects of the conditioned medium of SARS-CoV-2–infected hACE2-A549 cells and hypoxia on the expression of complement proteins in HLMVECs. HLMVECs were preexposed to normoxic or hypoxic (3% O2) conditions for 24 hours, followed by the addition of conditioned medium from mock-infected or SARS-CoV-2–infected hACE2-A549 cells for 24 hours. (A) Total RNA was isolated, and mRNA concentrations of C3 (complement C3), C3AR1 (complement C3a receptor 1), C1QA (complement C1q A chain), and CFB (complement factor B) were determined using qRT-PCR analysis. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and #P = 0.0508 (borderline significant). (B) Total cell lysates were collected and analyzed for the expression of indicated complement components using western blot analysis, and the representative blots are presented. (C) Densitometry of the blots in B is presented (technical replicates).
Vascular Pathology in COVID-19
COVID-19 is characterized by a progressive lung disease primarily targeting lung alveolar epithelial cells. However, clinical data and autopsies have shown that the disease is also associated with intravascular coagulation, endothelial dysfunction, and vascular involvement. This has been extensively documented in various past studies and even
COVID-19 News reports. Thrombotic microangiopathy, vasculitis, and angiogenesis have been observed in COVID-19 cases, highlighting the complexity of the disease and its impact on vascular health.
Endothelial Susceptibility to SARS-CoV-2 Infection
The study sought to determine whether lung endothelial cells and other vascular cells are susceptible to SARS-CoV-2 infection. ACE2 and TMPRSS2, critical cellular entry factors for SARS-CoV-2, were found to be expressed at significantly lower levels in lung vascular cells compared to alveolar epithelial cells. This reduced expression explained the limited susceptibility of vascular cells to productive SARS-CoV-2
infection. While some studies suggested the possibility of abortive infection in endothelial cells, no evidence of productive infection was found in lung microvascular endothelial cells (HLMVECs) or other resident vascular cells.
Hypoxia and Viral Uptake
As COVID-19 often leads to alveolar hypoxemia, researchers investigated whether hypoxia could potentiate endothelial responses to SARS-CoV-2 infection. While ACE2 and TMPRSS2 expression increased under hypoxic conditions in HLMVECs, no enhancement of SARS-CoV-2 infection was observed. Instead, a pattern of viral uptake without replication, referred to as abortive infection, was detected, indicating a possible mechanism by which endothelial cells could encounter viral material without supporting active viral replication.
Indirect Endothelial Activation
The study delved into the possibility of endothelial activation occurring as a result of paracrine factors released by SARS-CoV-2-infected epithelial cells. It was observed that exposure of HLMVECs to conditioned medium from infected cells led to upregulation of adhesion molecules and inflammatory cytokines, suggesting that viral particles released by damaged epithelial cells could indirectly contribute to endothelial inflammation and hyperpermeability.
Complement Activation and Inflammatory Signaling
The complement system, a component of the innate immune response, plays a role in COVID-19 pathology by mediating vascular inflammatory and thrombotic responses. Clinical data has demonstrated increased complement production in COVID-19 patients. The study revealed that exposure of HLMVECs to conditioned medium from infected cells, coupled with hypoxia, upregulated complement components and inflammatory markers. This indicated that a local endothelial complement system could be activated in response to viral exposure, further contributing to endothelial activation and inflammation.
Furthermore, the study demonstrated that exposure of HLMVECs to conditioned medium from SARS-CoV-2–infected human ACE2 stably transfected A549 epithelial cells and hypoxia resulted in upregulation of inflammatory factors such as ICAM-1 (intercellular adhesion molecule 1), VCAM-1 (vascular cell adhesion molecule 1), and IL-6 (interleukin 6) as well as complement components such as C3 (complement C3), C3AR1 (complement C3a receptor 1), C1QA (complement C1q A chain), and CFB (complement factor B). Taken together, the study data support a model in which lung endothelial and vascular dysfunction during COVID-19 involves the activation of complement and inflammatory signaling and does not involve productive viral infection of endothelial cells.
Implications and Future Directions
The findings of the study provide insights into the complex interplay between SARS-CoV-2 infection, endothelial dysfunction, and inflammation. The study confirms that endothelial cells are not productively infected by the virus but may uptake viral material without replication. Moreover, the study highlights the role of hypoxia in modulating endothelial responses to SARS-CoV-2 exposure. Indirect activation of endothelial cells by viral particles and the subsequent activation of the local complement system shed light on potential therapeutic strategies. The results of the study suggest that therapeutic strategies directed at strengthening the endothelial barrier, eliminating vascular inflammation, and complement activation could be helpful in ameliorating endothelial injury and cardiovascular complications in patients with COVID-19.
Conclusion
The research conducted at the University of Colorado Anschutz Medical Campus deepens our understanding of the intricate interactions between SARS-CoV-2, endothelial cells, and the vascular system. While endothelial cells are not primary targets of productive infection, they do play a crucial role in the pathogenesis of COVID-19 through indirect activation mechanisms. The study underscores the significance of local complement activation and inflammatory signaling in endothelial dysfunction and suggests potential avenues for therapeutic interventions aimed at alleviating endothelial injury and cardiovascular complications in patients with COVID-19. As the global effort to combat the pandemic continues, the insights gained from studies like this one contribute to our ability to effectively manage and treat COVID-19 patients.
The study findings were published in the peer reviewed American Journal of Respiratory Cell and Molecular Biology.
https://www.atsjournals.org/doi/full/10.1165/rcmb.2022-0373OC
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
