BREAKING! French Study Warns That COVID-19 mRNA Vaccines Can Cause Multisystemic Hyper-Inflammatory Syndrome In Children Aged 12-17!
Source: mRNA Vaccines-MIS-C Jan 20, 2022 4 years, 3 weeks, 3 days, 13 hours, 29 minutes ago
mRNA Vaccines-MIS-C: French researchers from various universities and pediatric specialists from over 25 hospitals in France have in a new study alarmingly found that COVID-19 mRNA vaccines can cause multisystemic hyper-inflammatory syndrome in children aged 12-17!
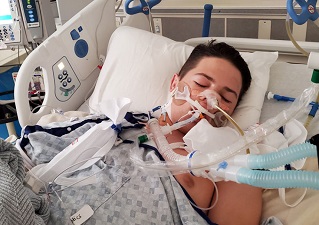
Multisystem inflammatory syndrome in children (MIS-C) is the most severe life-threatening clinical entity associated with pediatric SARS-CoV-2 infection.
Till this study, it was never known whether COVID-19 mRNA vaccine can induce this complication in children despite such occurrences being reported.
The
mRNA Vaccines-MIS-C study team wanted to assess the risk of hyper-inflammatory syndrome following COVID-19 mRNA vaccine in children.
The study involved the post-authorization national population-based surveillance using the French enhanced pharmacovigilance surveillance system for COVID-19 vaccines. All cases of suspected hyper-inflammatory syndrome following COVID-19 mRNA vaccine in 12-17-year-old children between June 15th, 2021 and January 1st, 2022, were reported. Each case was assessed for WHO MIS-C criteria.
Causality assessment followed 2019 WHO recommendations.
The main outcome was the reporting rate of post-vaccine hyper-inflammatory syndrome per 1,000,000 COVID-19 mRNA vaccine doses in 12-17-year-old children. This reporting rate was compared to the MIS-C rate per 1,000,000 12-17-year-old children infected by SARS-CoV-2. Secondary outcomes included the comparison of clinical features between post-vaccine hyper-inflammatory syndrome and post SARS-CoV-2 MIS-C.
The study findings showed that from June 2021 to January 2022, 8,113,058 COVID-19 mRNA vaccine doses were administered to 4,079,234 12-17-year-old children. Among them, 9 presented a multisystemic hyper-inflammatory syndrome. All cases fulfilled MIS-C WHO criteria.
Main clinical features included male predominance (8/9, 89%), cardiac involvement (8/9, 89%), digestive symptoms (7/9, 78%), coagulopathy (5/9, 54%), cytolytic hepatitis (4/9, 46%), and shock (3/9, 33%). 3/9 (33%) required intensive care unit transfer, and 2/9 (22%) hemodynamic support. All cases recovered. Only three cases had evidence of previous SARS-CoV-2 infection.
The reporting rate was 1.1 (95%CI [0.5; 2.1]) per 1,000,000 doses injected. As a comparison, 113 MIS-C (95%CI [95; 135]) occurred per 1,000,000 12-17-year-old children infected by SARS-CoV-2.
Clinical features (inflammatory parameters, cytopenia) slightly differed from post-SARS-CoV-2 MIS-C, along with short-term outcomes (less PICU transfer than MIS-C). Conclusion and Relevance. Very few cases of hyper-inflammatory syndromes with multi-organ involvement occurred following COVID-19 mRNA vaccine in 12-17-year-old children.
The low reporting rate of this syndrome, compared to the rate of MIS-C among same age children infected by SARS-CoV-2, supports the benefit of SARS-CoV-2 vaccination in children.
However, parents, teachers and pediatric doctors need to be aware of this adverse manifestation from mRNA COVID-19 vaccines among children.
Further studies are required to explore speci
fic pathways of this entity compared to post-SARS-CoV-2 MIS-C.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.medrxiv.org/content/10.1101/2022.01.17.22269263v1
MIS-C is a novel clinical entity characterized by shock, frequent acute cardiac dysfunction, and multi-organ failure requiring intensive care unit transfer of the patient and hemodynamic support. There is documented evidence that associates severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with MIS-C.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) In May 2021, authorized the use of the BNT162b2 mRNA COVID-19 vaccine in individuals of 12 to 17 years of age. However, the potential of the SARS-CoV-2 antigens in inducing rare conditions like MIS-C was never investigated.
The study team conducted a post-authorization prospective national population-based surveillance to investigate the association of coronavirus disease 2019 (COVID-19) mRNA vaccines with MIS-C in children aged 12 to 17 years in France.
All children under 18 years of age exhibiting fever for more than three days, shock, inflammatory syndrome, or unexplained acute organ dysfunction after receiving the COVID-19 mRNA vaccine in France from June 15, 2021, to January 1, 2022, were eligible for the study.
All these identified cases were assessed to verify if they fulfilled the World Health Organization (WHO) criteria for MIS-C. The cases fulfilling the criteria were further reviewed to include patients reporting a delay of fewer than two months between COVID-19 vaccination and the onset of MIS-C.
The expected primary outcome of the study was the national reporting rate of MIS-C after COVID-19 mRNA vaccination per 1,000,000 vaccine doses in 12 to 17-year-old children in France
The study team compared the national reporting rate with the rate of post-SARS-CoV-2 MIS-C cases per 1,000,000 infections in 12- to 17-year-old children. The reporting rate of MIS-C after the first and second COVID-19 mRNA vaccine doses in the same age group was considered the secondary outcome.
The study findings showed that nine cases of MIS-C were observed among the 2,028 COVID-19 mRNA vaccine-related adverse drug reactions reported. Complete data for history of documented COVID-19 infection, nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR), and anti-N serology were available for these MIS-C cases.
Importantly rhe onset of the disease was noted two to 42 days after the last vaccination dose was received.
The median age of most studied patients was 12.5 years, and 89% of patients were male, while three of the male patients had comorbidities like type 1 diabetes, osteochondritis with obesity, and leukemia. Clinical characteristics of MIS-C like cardiac involvement, gastrointestinal symptoms, coagulopathy, mucocutaneous involvement, cytolytic hepatitis, and shock were observed in the patients.
Interestingly, the characteristic manifestations of MIS-C, like the expansion of Vb21.3-expressing T cells, were not detected in the patients in the present study.
The study team noted a national reporting rate of 1.1 per 1,000,000 mRNA vaccine doses received by the study group of 12 to 17-year-old children. The national MIS-C reporting rate in children after the first mRNA vaccine dose was 1.2, while in children after the second mRNA dose vaccination, it was 1.0.
It was found that the reporting rate was lower in females as compared to males. In comparison, the reporting rate for the occurrence of MIS-C post-SARS-CoV-2 infection is 113.3 per 1,000,000 in 12-to 17-year-old infected children.
The discovered national reporting rate of 1.1 included cases with no recorded history of SARS-CoV-2 infection, suggesting a link between the COVID-19 mRNA vaccine and MIS-C. The rate of occurrence of MIS-C post-SARS-CoV-2 infection is 100 times higher than post-COVID-19 mRNA vaccination.
Clinical features of MIS-C like prolonged hyper-inflammatory state, involvement of multiple organs, and disease severity were observed to be common in both post-SARS-CoV-2-MIS-C occurrences and MIS-C post-COVID-19 mRNA vaccination.
Clinical and immunological divergences like the lack of expansion of a subset of T cells indicate a difference in clinical pathways of MIS-C manifestations, which need further investigation.
For more about
mRNA Vaccines-MIS-C, keep on logging to Thailand Medical News.
