BREAKING! SARS-CoV-2 Nucleocapsid Protein Exhibits RNA Annealing and Chaperone Activity That Surpasses HIV!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jul 05, 2025 7 months, 1 week, 1 day, 23 hours, 1 minute ago
Thailand Medical News: In a remarkable discovery that deepens our understanding of the SARS-CoV-2 virus, a new study has revealed that its nucleocapsid protein (N protein) possesses an exceptional ability to rearrange and manage RNA structures—a function previously thought to be dominated by HIV-1 nucleocapsid proteins. This groundbreaking research was led by scientists from Université Paris-Saclay’s Laboratoire de Biologie et Pharmacologie Appliquée (UMR 8113 CNRS, École Normale Supérieure de Paris-Saclay, France) and the College of Life Sciences at Northwest A&F University in Shaanxi, China.
 SARS-CoV-2 Nucleocapsid Protein Exhibits RNA Annealing and Chaperone Activity That Surpasses HIV
SARS-CoV-2 Nucleocapsid Protein Exhibits RNA Annealing and Chaperone Activity That Surpasses HIV
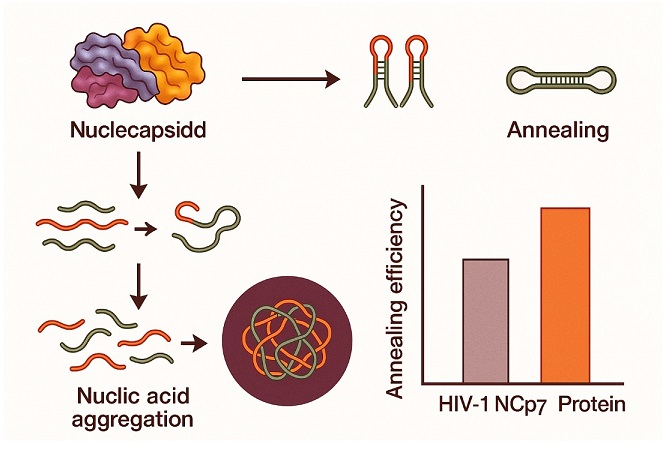
The study shows that the full-length SARS-CoV-2 N protein demonstrates a high level of nucleic acid chaperone activity, meaning it can efficiently assist in folding, unfolding, and rearranging RNA structures—critical steps in viral replication and genome packaging. In fact, this
Thailand Medical News report highlights that the N protein's annealing activity is not only comparable but often superior to that of HIV-1’s renowned nucleocapsid proteins, NCp7 and its precursor NCp9.
Unlocking the Hidden Powers of the Nucleocapsid Protein
The N protein has long been known to bind viral RNA and help compact it into new virus particles. But the new study goes beyond this basic understanding by demonstrating that the N protein actively promotes the annealing of complementary RNA hairpin loops—complicated structures essential for the virus to replicate and transcribe its genetic code.
Using multiple techniques—including Electrophoretic Mobility Shift Assays (EMSA) and fluorescence-based FRET experiments—the researchers found that the N protein could achieve annealing efficiency up to 73% in some assays. This surpasses the performance of HIV-1 NCp7 under similar laboratory conditions.
Importantly, the research revealed that only the full-length N protein, encompassing all five of its domains, is highly effective at this task. Truncated versions of the protein, missing key structural regions, showed much weaker RNA annealing ability. The dimerization domain in the C-terminal region appears particularly vital, as it allows the protein to form pairs that facilitate efficient RNA handling.
Speed, Strength, and Structure
In kinetic analyses, the N protein was shown to enable RNA hybridization through both a fast and a slower phase. The slower phase, which reflects the protein’s ability to fully stabilize and extend RNA duplexes, was particularly enhanced at acidic pH levels—indicating that the protein works better in environments that promote its oligomerization (formation of multi-unit protein complexes).
Interestingly, the protein also demonstrated the ability to promote the aggregation of nucleic acids—clumping RNA strands together—which is another key feature of effective chape
rone proteins. The aggregation ability of the SARS-CoV-2 N protein was even greater than that of HIV-1 NCp9, reinforcing its potent chaperone characteristics.
However, the protein was less effective at destabilizing short, stable double-stranded RNA segments. This suggests that while it excels at promoting annealing, its unwinding ability may be limited to certain RNA lengths or structures—leaving other viral proteins like helicases to perform that specific function.
Why This Matters
These findings have far-reaching implications. RNA chaperone activity is crucial for viruses like SARS-CoV-2 to replicate rapidly and evade host defenses. By facilitating template switching and the reorganization of viral RNA, the N protein plays a central role in both continuous and discontinuous transcription—key steps in the virus’s life cycle. The study also suggests that this activity could be involved in packaging the viral genome and possibly even in its interactions with host cell microRNAs.
Most notably, the N protein’s chaperone capabilities may help explain why newer SARS-CoV-2 variants, such as Delta and Omicron, demonstrate increased infectivity—both of which carry mutations in the N protein that could enhance its RNA-handling abilities.
Conclusion
This new research positions the SARS-CoV-2 nucleocapsid protein as one of the most efficient RNA chaperones observed in virology, rivaling or even exceeding HIV-1’s established proteins. By enabling rapid and efficient RNA annealing, the N protein is likely crucial for the virus’s replication and packaging processes. Understanding this function opens potential pathways for therapeutic targeting—especially if future drugs can disrupt these RNA-protein interactions and hinder the virus’s ability to reproduce and spread.
The study findings were published in the peer reviewed Journal of Molecular Biology.
https://www.sciencedirect.com/science/article/pii/S002228362500378X
For the latest COVID-19 News, keep on logging to
Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-covid-19-news-study-shows-that-sars-cov-2-nucleocapsid-protein-has-dna-melting-and-strand-annealing-properties
https://www.thailandmedical.news/news/sars-cov-2-nucleocapsid-protein-prevents-viral-degradation-by-disrupting-nonsense-mediated-mrna-decay
https://www.thailandmedical.news/news/sars-cov-2-nucleocapsid-protein-affects-cellular-defense-by-targeting-the-nonsense-mediated-mrna-decay-nmd-pathway
