COVID-19 News: Study Discovers That SARS-CoV-2 ORF8 Protein Exhibits Complement Inhibitory Properties And Damages Innate Immunity!
COVID-19 News - ORF8 Inhibits Complement System Jan 25, 2023 3 years, 4 weeks, 1 day, 18 hours, 38 minutes ago
COVID-19 News: Researchers from the Neurobiology and Drug Discovery (NDD) Laboratory, Department of Molecular Medicine (DMM), Jamia Hamdard University, New Delhi-India and the School of Biosciences and Bioengineering, Indian Institute of Technology Mandi, Himachal Pradesh- India have discovered that the SARS-CoV-2 encoded ORF8 protein exhibits complement inhibitory properties and as a result disrupts and damages innate immunity!
The complement system, also known as complement cascade, is a part of the innate immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and attack the pathogen's cell membrane.
It is a critical part of the innate immune system, which is not adaptable and does not change during an individual's lifetime. The complement system can, however, be recruited and brought into action by antibodies generated by the adaptive immune system.
The complement system consists of a number of small proteins that are synthesized by the liver, and circulate in the blood as inactive precursors. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this complement activation or complement fixation cascade is stimulation of phagocytes to clear foreign and damaged material, inflammation to attract additional phagocytes, and activation of the cell-killing membrane attack complex. About 50 proteins and protein fragments make up the complement system, including serum proteins, and cell membrane receptors. They account for about 10% of the globulin fraction of blood serum.
Three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway. The alternative pathway accounts for the majority of terminal pathway activation and so therapeutic efforts in disease have revolved around its inhibition.
In our past
COVID-19 News coverages, we had shown how SARS-CoV-2 induced activation of the complement system results in disease severity.
https://www.thailandmedical.news/news/covid-19-research-complement-cascade-of-immunity-system-and-coagulation-dysfunction-are-what-triggers-covid-19-severity-outcomes
https://www.thailandmedical.news/news/breaking-sars-cov-2-induced-antibody-mediated-complement-activation-and-subsequent-cellular-priming-exacerbates-covid-19-severity
https://www.thailandmedical.news/news/german-study-shockingly-discov
ers-that-sars-cov-2-caused-complement-activation-induces-excessive-cd16-t-cells-with-increased-cytotoxic-functions
https://www.thailandmedical.news/news/-sars-cov-2-upregulates-cd55-complement-regulator-and-prolongs-its-activation-causing-an-immune-dysfunction-that-contributes-to-disease-severity-in-so
Though the tightly controlled complement pathways are regarded as major innate defense mechanism of the host, accidental dysregulation of any of the components leading to hyperactivation of the complement system can cause devastating damage to the host tissues.
Hyperactivation of the complement system, a major component of innate immunity and tissue damaging complement activation has been demonstrated to be the hallmark of pathophysiology in large section of COVID-19 patients.
https://pubmed.ncbi.nlm.nih.gov/34289657/
https://pubmed.ncbi.nlm.nih.gov/32943538/
Latest study findings implicate both the spike and nucleocapsid proteins of SARS-CoV-2 in the activation of the lectin as well as the alternative complement pathways.
https://pubmed.ncbi.nlm.nih.gov/34912108/
https://pubmed.ncbi.nlm.nih.gov/35839316/
Hence, complement activation in COVID-19 patients appears to be more of a bystander effect rather than by design to gain advantage by the virus in the host.
Thus, a major question remained unanswered so far about how SARS-CoV-2 escapes the complement system surveillances despite its robust activation.
In order to counter the complement-induced host defense system, viruses have also evolved unique strategies to escape this surveillance network.
Frequent instances are documented where the viruses have adopted molecular mimicry by encoding orthologs of the complement family of proteins or co-opting the host complement system to gain evolutionary advantage.
https://pubmed.ncbi.nlm.nih.gov/28670306/
https://journals.asm.org/doi/full/10.1128/JVI.00668-17
The study team hence investigated whether similar strategies are also adopted by SARS-CoV-2 to bypass activated complement pathways.
During activation, complement alternative pathway (AP) converge on cleaving the complement component C3 into C3a and C3b fragments by a functional C3-convertase (C3bBb) complex. Activation of factor B (FB) by Factor D (FD) is a prerequisite for C3-converatse formation. C3b acts as a major opsonin tagging the pathogen surface and promoting assembly of the convertases, leading to its amplification and targeted clearance of the pathogen. Factor I (FI) mediated cleavage of C3b acts as a critical negative-feedback regulatory arm of the AP.
The study findings demonstrated the interaction of SARS-CoV-2 encoded ORF8 protein with C3b and unravel the molecular mechanism of this binding.
The study findings identified SARS-CoV-2-encoded open reading frame 8 (ORF8) protein as one of the major binding partners of human complement C3/C3b components and their metabolites.
The study findings demonstrated that pre-incubation of ORF8 with C3/C3b in the fluid phase has two immediate functional consequences in the alternative pathway (AP); this pre-incubation inhibits factor I (FI)-mediated proteolysis and blocks factor B (FB) zymogen activation into active Bb. ORF8 binding results in occlusion of both factor H (FH) and FB from C3b, rendering the complexes resistant to FI-mediated proteolysis and inhibition of pro-C3-convertase (C3bB) formation, respectively.
The study findings also confirmed the complement inhibitory activity of ORF8 in our hemolysis-based assay, where ORF8 prevented human serum-induced lysis of rabbit erythrocytes with an IC50 value of about 2.3 μM.
This inhibitory characteristic of ORF8 was also supported by in-silico protein-protein docking analysis, as it appeared to establish primary interactions with the β-chain of C3b, orienting itself near the C3b CUB (C1r/C1s, Uegf, Bmp1) domain like a peptidomimetic compound, sterically hindering the binding of essential cofactors required for complement amplification. Thus, ORF8 has characteristics to act as an inhibitor of critical regulatory steps in the AP, converging to hasten the decay of C3-convertase and thereby, attenuating the complement amplification loop.
The study findings were published in the peer reviewed Journal OF Biological chemistry.
https://www.jbc.org/article/S0021-9258(23)00062-5/fulltext
The study findings provide evidence that SARS-CoV-2 ORF8 protein plays a major role in chocking AP by interacting with C3/C3b and shielding major complement factors from binding. ORF8 interacts with C3b in such an optimized conformation that it not only prevents proteolysis by FI but also hinders activation of FB for functional C3-convertase formation. By cleaving C3b, FI hastens the decay of C3-converatase formation preventing hyperactivation and tissue damage. The binding of ORF8 to C3b appears to precede the binding of FH and recruitment of FB, resulting in blocking some of these essential functions in AP and allowing the virus to escape the detection and clearance.
The ORF8 protein has been shown to exist both as homodimer and monomer.
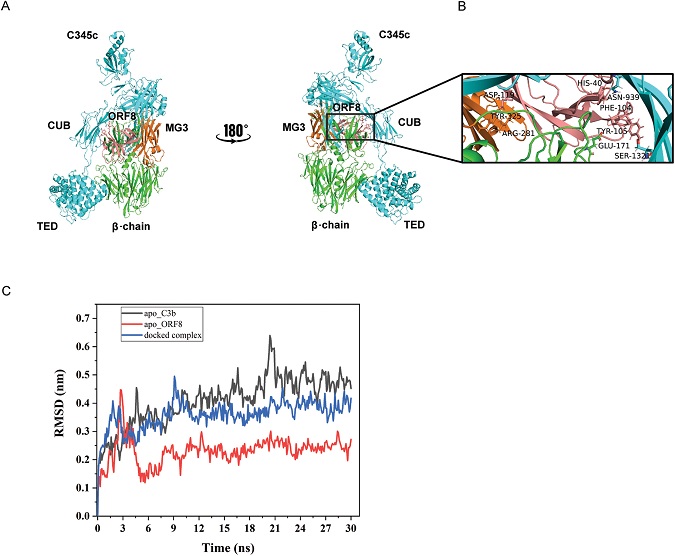 In-silico model of C3b-ORF8 co-complex. (A) Mini FH and FI were replaced from the structure C3b-mini FH-FI structure (PDB entry 5O32) and docked with ORF8 (PDB entry7JTl). ORF8 (salmon) appears to partially occupy complement control protein (CCP) binding domain 2-3 on C3b. ORF8 is shown to make multiple contacts with the MG3 (orange) and MG2 domains of β-chain. (B) Zoomed view of interactions between the MG3 domain residues (orange), ARG-281 and TYR-325 with ASP-119 (ORF8). Also, interactions between His-40 (ORF8)/ASP-939 (α-chain C3b) and PHE-104 (ORF8) / GLU-171 (β-chain C3b) respectively, show proximity to the second cleavage site RS (1320-1321) of CUB domain in α-chain (cyan) of C3b. More information in supplementary Table S1. (C) Molecular Dynamics simulation analysis confirms the stabilization of the docked complex (C3b-ORF8) as compared to the apo-C3b (un-complexed). Root Mean Square Deviation (RMSD) values (nm) generated for 30 ns at 150 mM KCl solvent system are plotted for comparison using Gromacs simulation package.
In-silico model of C3b-ORF8 co-complex. (A) Mini FH and FI were replaced from the structure C3b-mini FH-FI structure (PDB entry 5O32) and docked with ORF8 (PDB entry7JTl). ORF8 (salmon) appears to partially occupy complement control protein (CCP) binding domain 2-3 on C3b. ORF8 is shown to make multiple contacts with the MG3 (orange) and MG2 domains of β-chain. (B) Zoomed view of interactions between the MG3 domain residues (orange), ARG-281 and TYR-325 with ASP-119 (ORF8). Also, interactions between His-40 (ORF8)/ASP-939 (α-chain C3b) and PHE-104 (ORF8) / GLU-171 (β-chain C3b) respectively, show proximity to the second cleavage site RS (1320-1321) of CUB domain in α-chain (cyan) of C3b. More information in supplementary Table S1. (C) Molecular Dynamics simulation analysis confirms the stabilization of the docked complex (C3b-ORF8) as compared to the apo-C3b (un-complexed). Root Mean Square Deviation (RMSD) values (nm) generated for 30 ns at 150 mM KCl solvent system are plotted for comparison using Gromacs simulation package.
The in-silico results predict the c-terminal portion of the monomeric region, containing antiparallel β-strands linked by a hairpin-loop structural feature, to fold into the C3b central core of the β-chain. These interactions, mostly with MG3 domain of the β-chain, orient the remaining part of the ORF8 molecule to occupy the surface near the CUB domain perhaps blocking the FH-FI binding. A C3b interacting protein CRIg (complement receptor of the immunoglobulin superfamily; also called V-set and immunoglobulin domain-containing protein 4) has been known to interact primarily with the β-chain and reported to act as an inhibitor of convertase activity.
Interestingly, the ORF8 protein appears to have sequence resemblance not only with CRIg (about 39% similarity) but also with the c-terminal region of FI protein sequence. The combinations of limited sequence resemblance with specific domains of known C3-convertase down-regulators like FI and CRIg perhaps allows it to get access to the central β-core of various conformational states of C3b, rendering it inaccessible to the co-factors FH and FB, as demonstrated experimentally.
Opsonization by C3b is believed to play a central role in protection of host against pathogens and its clearance by the complement system. Hence, viral and bacterial pathogens have evolved strategies to effectively target C3b specifically to gain evolutionary advantage.
These strategies however seem to be microbe specific. Herpes virus, being the first classical example, uses one of the several glycoproteins (gC1) on the viral surface to bind with C3b resulting in its inactivation and accelerating the decay of C3b convertase activity.
https://pubmed.ncbi.nlm.nih.gov/3018078/
Viruses like Nipah, on the other hand uses protease activity reminiscent of FI protease to enhance degradation of C3b, thus blocking the complement activation via reduced C3-converatse formation.
https://pubmed.ncbi.nlm.nih.gov/25355897/
In contrast, the staphylococcal complement inhibitor (SCIN) act as a competitive inhibitor occluding FH binding.
https://pubmed.ncbi.nlm.nih.gov/19625656/
In the same way, the ORF8 proteins of SARS-CoV-2 appears to target critical steps of AP by interacting with C3b. These steps include occlusion of FH as evident from FI inhibition and prevention of FB activation. Thus, ORF8 appears to function more like SCIN in inhibiting AP.
The research findings provide direct evidence of binding of SARS-CoV-2 ORF8 protein to complement components C3/C3b, primarily making them resistant to binding to other major co-factors required for activation and regulation of the AP pathway. Though several questions remain unanswered at this stage in the context of ORF8-mediated physiological regulation of complement C3 activation, the study findings provide a unique framework to unravel further insights into the role of ORF8 in subverting complement surveillance.
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-news-scientist-identify-antibody-ncov396-that-halts-sars-cov-2-nucleocapsid-protein-induced-complement-hyperactivation-
https://www.thailandmedical.news/news/covid-19-latest-study-identifies-factor-d-inhibitor-ach-145951-that-can-inhibit-direct-activation-of-the-alternative-complement-pathway-by-sars-cov-2-
https://www.thailandmedical.news/news/breaking-narsoplimab-may-prevent-and-treat-covid-19-severity-by-halting-complement-cascade-says-researchers

