Stanford Study Uncovers Yet Another Way That The SARS-CoV-2 Suppresses Host Immune Response, This Time By The Virus Proteases Cleaving NEMO!
Source: Medical News-SARS-CoV-2-NEMO Nov 17, 2021 4 years, 3 months, 1 week, 14 hours, 6 minutes ago
Researchers from Stanford University along with scientists from Oak Ridge National Laboratory, University of Kentucky, Chapman University School of Pharmacy-Irvine, University of Tennessee Knoxville and the US Department of Energy have in a new study uncovered yet another manner in which the SARS-CoV-2 coronavirus suppresses the human host immune system, this time via the virus proteases cleaving the a human immune signaling protein called NF-kB Essential Modulator or NEMO.
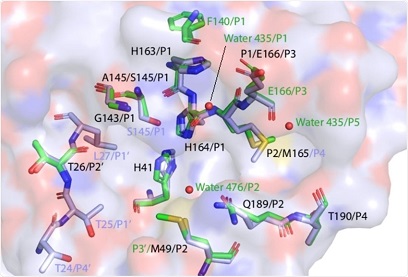 Comparison of 3CLpro interactions with its N-terminal sequence and NEMO
Comparison of 3CLpro interactions with its N-terminal sequence and NEMO
The study finding showed that in addition to its essential role in viral polyprotein processing, the SARS-CoV-2 3C-like (3CLpro) protease can cleave human immune signaling proteins, like NF-κB Essential Modulator (NEMO) and deregulate the host immune response.
During the study, in vitro assays showed that SARS-CoV-2 3CLpro cleaves NEMO with fine-tuned efficiency. Analysis of the 2.14 Å resolution crystal structure of 3CLpro C145S bound to NEMO226-235 reveals subsites that tolerate a range of viral and host substrates through main chain hydrogen bonds while also enforcing specificity using side chain hydrogen bonds and hydrophobic contacts. Machine learning- and physics-based computational methods predict that variation in key binding residues of 3CLpro-NEMO helps explain the high fitness of SARS-CoV-2 in humans.
The study team posit that cleavage of NEMO is an important piece of information to be accounted for in the pathology of COVID-19.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.11.11.468228v1
The study findings highlighted a novel function of 3C-like protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus protease has been found to cleave a human immune signaling protein called NF-kB Essential Modulator (NEMO) and disrupt the host immune response.
Already it has been found through various studies that the non-structural proteins of SARS-CoV-2, including papain-like protease and 3C-like protease, play essential roles in regulating viral lifecycle and disrupting host immune defense.
Importantly the 3C-like protease recognizes a specific motif in viral polyproteins and cleaves them to form mature non-structural proteins. The same recognition motif is also present in some proteins of the host innate immune pathways.
Alarmingly this indicates that 3C-like protease can cleave host proteins to disrupt the first-line antiviral defense mechanism.
The study team investigated whether 3C-like protease has any impact on human immune signaling protein NEMO. During canonical NF-kB response signaling, NEMO cleaves and activates NF-kB, which is an important step for early-stage viral clearance.
The research findings discovered that 3C-like protease interacts with Lys226-His235 of the α-helical Hlx2 domain of one NEMO protomer for cleavage. Thus, as mentioned by the study team, the protease might utilize a transient state of the α-helical domain for proteolysis. In addition, it might actively outcompete NEMO dimer interactions and unwind the α-h
elix.
Importantly it was found that the 3C-like protease formed hydrophobic interactions and hydrogen bonds with specific residues in the NEMO to outcompete the interactions between these residues in the NEMO dimer.
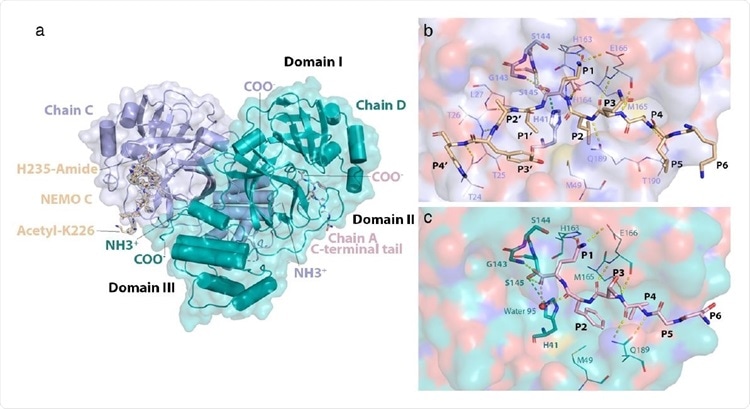 Structure of the NEMO-bound 3CLpro C145S homodimer. a) Global view of NEMObound 3CLpro dimer. Chains C and D have their N- (NH3+) and C-termini (COO-) labeled. NEMO bound into chain C (NEMO C) is displayed as sticks and colored wheat. A mesh of electron density is displayed around NEMO C in light grey. Acetylated Lys226 and amidated His235 at the N- and C-terminus of the NEMO peptide, respectively, are labeled. The C-terminal tail of chain A is colored in light pink, displayed as sticks and is observed to bind into the substrate-binding site of chain D. Domains I (aa. 8-101), II (aa. 102-184) and III (aa. 201-303) are labeled in black. b) Interactions of NEMO with the substrate-binding groove of 3CLpro C145S. NEMO is depicted in wheat. Residues Lys226 to His235 of NEMO are labeled P6 to P4’, respectively. The surface of chain C is shown in light blue and colored by atom type, where oxygens are red, nitrogens are dark blue, carbons are light blue, and sulfurs are yellow. Residues in the substrate-binding site of chain C are portrayed as lines and labeled. Catalytically relevant residues22 in chain C are portrayed as sticks and also labeled in bold. Hydrogen bonds are depicted as dashed yellow lines. The hydrogen bond that would form between Cys145 (in WT) or Ser145 (in C145S) and His41 is depicted as a dashed green line. c) Interactions of the C-terminal tail of chain A with the substrate-binding groove of 3CLpro C145S. The C-terminal tail of chain A is depicted as sticks in light pink. Residues Ser301 to Gln306 are labeled P6 to P1 respectively. The surface of chain D is shown in teal and colored by atom type. Residues in the substrate-binding groove of chain D that interact with the C-terminal tail of chain A are portrayed as lines and labeled. Catalytically relevant residues and hydrogen bonds are portrayed as in (b). The oxygen atom of water 95 is depicted as a red sphere.
Structure of the NEMO-bound 3CLpro C145S homodimer. a) Global view of NEMObound 3CLpro dimer. Chains C and D have their N- (NH3+) and C-termini (COO-) labeled. NEMO bound into chain C (NEMO C) is displayed as sticks and colored wheat. A mesh of electron density is displayed around NEMO C in light grey. Acetylated Lys226 and amidated His235 at the N- and C-terminus of the NEMO peptide, respectively, are labeled. The C-terminal tail of chain A is colored in light pink, displayed as sticks and is observed to bind into the substrate-binding site of chain D. Domains I (aa. 8-101), II (aa. 102-184) and III (aa. 201-303) are labeled in black. b) Interactions of NEMO with the substrate-binding groove of 3CLpro C145S. NEMO is depicted in wheat. Residues Lys226 to His235 of NEMO are labeled P6 to P4’, respectively. The surface of chain C is shown in light blue and colored by atom type, where oxygens are red, nitrogens are dark blue, carbons are light blue, and sulfurs are yellow. Residues in the substrate-binding site of chain C are portrayed as lines and labeled. Catalytically relevant residues22 in chain C are portrayed as sticks and also labeled in bold. Hydrogen bonds are depicted as dashed yellow lines. The hydrogen bond that would form between Cys145 (in WT) or Ser145 (in C145S) and His41 is depicted as a dashed green line. c) Interactions of the C-terminal tail of chain A with the substrate-binding groove of 3CLpro C145S. The C-terminal tail of chain A is depicted as sticks in light pink. Residues Ser301 to Gln306 are labeled P6 to P1 respectively. The surface of chain D is shown in teal and colored by atom type. Residues in the substrate-binding groove of chain D that interact with the C-terminal tail of chain A are portrayed as lines and labeled. Catalytically relevant residues and hydrogen bonds are portrayed as in (b). The oxygen atom of water 95 is depicted as a red sphere.
The research findings identified specific subsites that contribute to the substrate versatility of 3C-like protease. Specific interactions of 3C-like protease with its N-terminal autoprocessing sequence or NEMO substrate were identified.
The study findings revealed that the S1 subsite is flexible and binds to the NEMO substrate through hydrophobic interactions.
However, this subsite did not interact with the N-terminal substrate. In contrast, the S5 subsite interacted with the N-terminal substrate by recruiting a water molecule.
Interestingly the interactions of S2 subsite with N-terminal and NEMO substrates were found to be conserved. However, the S3 and S4 subsites were identified as significant contributors to substrate versatility.
The study findings of the computational analysis revealed that the single substitution S46A in the substrate-binding site of 3C-like protease increases local rigidity, leading to a reduction in NEMO affinity.
Similarly, it was predicted that another substitution mutation V232A in NEMO can impair its interaction with 3C-like protease.
The detailed enzymatic assay findings revealed that cleavage of NEMO by 3C-like protease is a slow process and does not have a significant functional impact in the initial hours of infection.
Moreover, it has been observed in other studies that SARS-CoV-2 proteases cleave multiple host proteins, leading to a significant reduction in protein levels within 24 to 48 hours of infection.
The study findings overall indicate that 3C-like protease-mediated cleavage of NEMO is a part of various other mechanisms that are triggered by SARS-CoV-2 to suppress host immune responses.
Considering the clinical similarities between NEMO ablation and COVID-19, it could be considered that 3C-like protease-mediated cleavage of NEMO plays a significant role in COVID-19 pathophysiology.
Corresponding author, Dr Daniel Jacobson from the Biosciences Division, Oak Ridge National Laboratory told Thailand
Medical News, “The study identifies the role of SARS-CoV-2 3C-like protease in cleaving host immune protein NEMO. It is known that NEMO plays a central role in the mitochondrial antiviral-signaling protein-mediated antiviral immune response. Furthermore, ablation of NEMO is known to cause the inactivation of NF-kB and type 1 interferon response.”
Considering these study findings, the research team suggest that 3C-like protease can be considered as a potential therapeutic target for preventing virus-induced host immunosuppression as well as viral replication.
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.

