COVID-19 News: SARS-CoV-2 ORF7a Blocked Autophagy Flux By Intervening In The Fusion Between Autophagosome And Lysosome To Promote Viral Infection!
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 05, 2023 2 years, 3 months, 1 week, 2 days, 3 hours, 31 minutes ago
COVID-19 News: The world has been grappling with the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). As the virus continues to pose a significant threat to public health, scientists worldwide are working tirelessly to unravel the mysteries of this viral invader. One of the key areas of research pertains to the mechanisms by which the virus infiltrates the human body and evades the immune system. In this pursuit, the spotlight shines on SARS-CoV-2 ORF7a, a crucial open reading frame (ORF) in the infection and pathogenesis process. This
COVID-19 News report delves into a recent study by researchers from Affiliated Hospital of Southern Medical University, Shenzhen-China, Affiliated Cancer Hospital of Chengdu Medical College),Sichuan-China, Chengdu Medical College-China, Inner Mongolia Minzu University, Tongliao-China and Northwestern Polytechnical University, Xi'an-China that detailed the intricate world of SARS-CoV-2 ORF7a and its impact on autophagy, ultimately shedding light on potential therapeutic avenues to combat COVID-19.
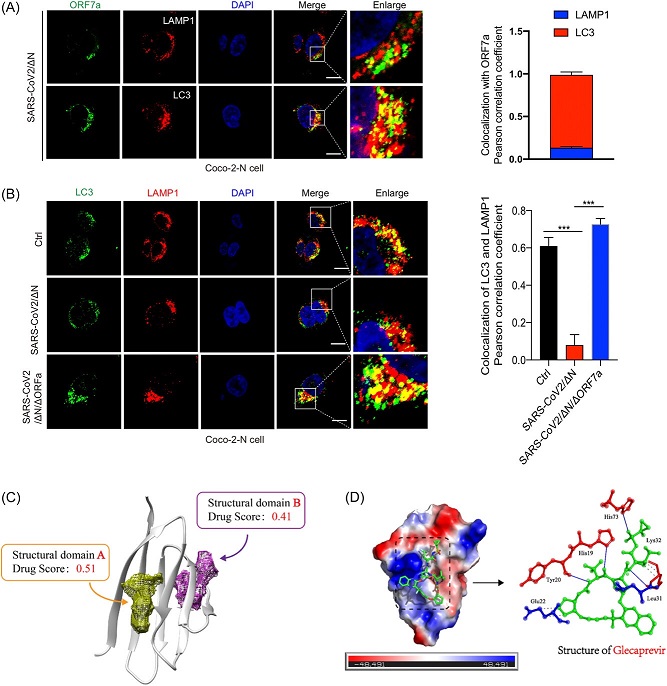 SARS-CoV-2 infection prevented autophagy flux, and Glecaprevir may be a potential drug against by targeting ORF7a. (A) Representative images of endogenous ORF7a and LAMP1 or LC3 staining in the SARS-CoV-2 infection Caco-2-N cell line. Pearson correlation coefficient is used to quantify the colocalization of ORF7a and LC3 or LAMP1. (B) Representative images of LAMP1 and LC3 staining of SARS-CoV-2/ΔN and SARS-CoV-2/ΔN/ΔORF7a trVLPs infected Caco-2-N cell line and control panel. Pearson correlation coefficient is used to quantify the colocalization of LC3 or LAMP1. (C) The two pocket structures of SARS-CoV-2 ORF7a. (D) The simulative combination of Glecaprevir with ORF7a pocket A. ORF7a, open reading frame-7a; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Understanding SARS-CoV-2 and Its Unique Infectious Nature
SARS-CoV-2 infection prevented autophagy flux, and Glecaprevir may be a potential drug against by targeting ORF7a. (A) Representative images of endogenous ORF7a and LAMP1 or LC3 staining in the SARS-CoV-2 infection Caco-2-N cell line. Pearson correlation coefficient is used to quantify the colocalization of ORF7a and LC3 or LAMP1. (B) Representative images of LAMP1 and LC3 staining of SARS-CoV-2/ΔN and SARS-CoV-2/ΔN/ΔORF7a trVLPs infected Caco-2-N cell line and control panel. Pearson correlation coefficient is used to quantify the colocalization of LC3 or LAMP1. (C) The two pocket structures of SARS-CoV-2 ORF7a. (D) The simulative combination of Glecaprevir with ORF7a pocket A. ORF7a, open reading frame-7a; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Understanding SARS-CoV-2 and Its Unique Infectious Nature
SARS-CoV-2 belongs to the family of beta coronaviruses, with a genome length of approximately 30 kilobases. While previous coronavirus outbreaks like SARS and MERS left their mark, SARS-CoV-2 has proven to be exceptionally infectious. Central to its infectivity is the spike (S) protein of SARS-CoV-2, which can effectively bind to the angiotensin I converting enzyme (ACE2). This interaction serves as a critical mechanism that makes the virus highly contagious and adaptable to its human host. The rapid spread of the virus, with nearly 200,000 daily infections at its peak, has outpaced global vaccination efforts. Unfortunately, effective drugs to treat COVID-19 remain elusive.
Autophagy-Lysosome Pathway: A Natural Defense Mechanism
Within the complex landscape of eukaryotic cells, the autophagy-lysosome pathway acts as a natural defense mechanism, efficiently eliminating invasive pathogens. SARS-CoV-2 has evolved several proteins to accelerate the formation of progeny viruses, effectively avoiding the host cell's clearance mechanisms. Nonstructural proteins (NSPs) and open reading frames (ORFs) are key players in this evasion strategy. Double membrane vesicles (DMVs) are pivotal in virus replication, protecting vir
al proteins and genetic material from degradation. DMVs, which are smaller and more abundant compared to typical autophagosomes, can originate from various cellular structures, including phagophores, omegasomes, autophagosomes, and the endoplasmic reticulum (ER)-associated degradation machinery.
Recent studies have highlighted how SARS-CoV-2 manipulates the autophagy-lysosome pathway to facilitate its infection and pathogenesis. Proteins like NSP3, NSP4, and NSP6 have been found to promote the formation of DMVs around the ER. The critical factor is that when NSPs are located on the DMVs' surface, they prevent these vesicles from fusing with lysosomes, a vital step for viral replication and evasion. ORFs, too, have been implicated in co-opting the autophagy-lysosome pathway for their advantage. For instance, ORF3a has been shown to obstruct the fusion of autophagosomes and lysosomes by interfering with the HOPS-RAB7 and VPS41-VPS39 complexes. This disruption promotes the accumulation of SARS-CoV-2 progeny viruses within the host. Additionally, ORF3a can disrupt ER homeostasis, inducing ER stress and inflammatory responses.
The Intricate Involvement of SARS-CoV-2 ORF7a
Many latest studies have drawn attention to the role of SARS-CoV-2 ORF7a in immune evasion and virus replication. Deletion of ORF7a has been shown to reduce the virulence of variant strains and the rate of progeny virus production. This protein has also been found to downregulate SNAP29, promoting viral replication. Furthermore, ORF7a can counteract the antiviral effects of SERINC5 by blocking its incorporation into budding virions. It also employs the ubiquitin system to evade host defenses through the PERK-elF2α-CHOP pathway. Interestingly, it has been reported that ORF7a is critical in blocking autophagy, which, in turn, hinders virus clearance.
However, the full extent of ORF7a's functions and its immune evasion mechanisms remained unclear, making it imperative to unravel the role and regulation of SARS-CoV-2 ORF7a during infection.
Research Insights: Decrypting SARS-CoV-2 ORF7a
The study we are discussing embarked on a comprehensive exploration of SARS-CoV-2 ORF7a's functions, aiming to shed light on its biological role and regulatory mechanisms during infection. The research took a multi-faceted approach, including the analysis of ORF7a's amino acid sequence, prediction of a three-dimensional crystal structure model of its transmembrane domain, and an investigation into its subcellular localization. The ultimate goal was to unravel the biological functions and mechanisms orchestrated by ORF7a.
One of the significant findings of this study was that SARS-CoV-2 ORF7a acts as a transmembrane protein, as revealed by a detailed analysis of its amino acid sequence. This characteristic contributes to its role in viral infection and pathogenesis.
Furthermore, the researchers unveiled that ORF7a plays a pivotal role in interfering with the autophagy-lysosome pathway through its interaction with p62, a crucial component of the autophagic machinery. This disruption of the pathway promotes the formation of double membrane vesicles, allowing the virus to evade host autophagy-lysosome degradation and innate antiviral immunity.
Such findings emphasize the importance of ORF7a as a potential therapeutic target in the fight against COVID-19.
Additionally, this study demonstrated that ORF7a prevents autophagic flux by blocking the fusion between autophagosomes and lysosomes. This insight not only enhances our understanding of the virus's mechanisms but also provides a potential avenue for drug development.
The Promise of Glecaprevir as a Targeted Therapeutic
One of the most promising aspects of this research is the identification of Glecaprevir as a potential drug for targeting SARS-CoV-2 ORF7a. Through structural analysis and antiviral drug screening, the study suggests that Glecaprevir may act as a structurally competitive inhibitor of the ORF7a domain. Glecaprevir is known as an inhibitor of the NS3/4A protease, which is located in the endoplasmic reticulum and plays a crucial role in the modification and expression of viral proteins. It has been reported that NS3/4A protease is a key regulatory protein during virus infection and replication, with involvement in endoplasmic reticulum stress and host cell autophagy. Inhibiting the activity of NS3/4A protease can significantly impact the expression of interferons (IFN), further highlighting its relevance in the battle against COVID-19.
Implications and Future Directions
In conclusion, while vaccination efforts against SARS-CoV-2 have made significant strides, the search for effective drugs to treat COVID-19 remains an urgent priority. The comprehensive understanding of ORF7a's functions, as revealed in this study, may prove invaluable in the development of novel therapies and clinical drugs against COVID-19. Glecaprevir, with its potential to target SARS-CoV-2 ORF7a, presents a promising avenue for drug development.
The research outlined in this article represents another significant step in our quest to combat the COVID-19 pandemic. As the scientific community continues to collaborate and innovate, the future holds hope for more effective treatments and ultimately the end of this global health crisis. The study of SARS-CoV-2 ORF7a and its role in autophagy represents a beacon of light in our ongoing battle against the virus, offering a potential path towards a healthier, safer world.
The study findings were published in the peer reviewed Journal of Medical Virology.
https://onlinelibrary.wiley.com/doi/10.1002/jmv.29200
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-shows-that-transmembrane-domains-play-an-important-role-in-bst-2-and-sars-cov-2-orf7a-interactions
https://www.thailandmedical.news/news/yale-study-discovers-that-sars-cov-2-proteins-orf7a-and-orf3a-downregulates-mhc-i-expression-aiding-in-immune-evasion-and-immune-dysfunction
