BREAKING COVID-19 News! Texas Study Finds That Omicron Variants Utilize Conformational Changes To Evade Neutralization By Key Antibodies!
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 18, 2023 2 years, 2 months, 6 days, 16 hours, 50 minutes ago
COVID-19 News: The relentless evolution of the SARS-CoV-2 virus has posed unprecedented challenges in our ongoing battle against the COVID-19 pandemic. A recent groundbreaking study covered in this
COVID-19 News report, conducted by the Center for Systems and Synthetic Biology at the University of Texas at Austin has delved into the intricacies of the Omicron variant, unraveling its unique ability to evade neutralization by key antibodies. This research sheds light on the intricate interplay between viral mutations and the host immune response, offering crucial insights for the development of more effective vaccines and therapeutic strategies.
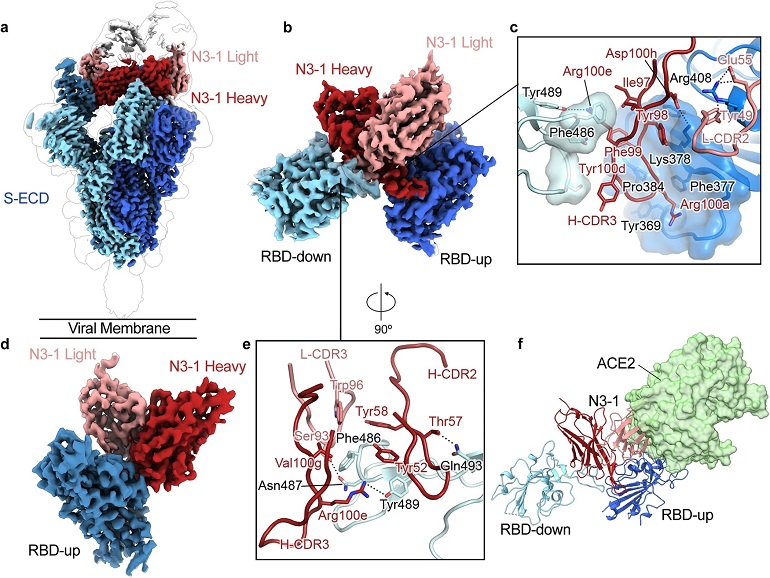 RBD-directed mAb N3-1 exhibits a unique binding mode by recognizing two distinct epitopes.
RBD-directed mAb N3-1 exhibits a unique binding mode by recognizing two distinct epitopes.
a Composite cryo-EM map of N3-1-bound SARS-CoV-2 Wuhan-Hu-1 spike. Each protomer is depicted in steel blue, royal blue and sky blue. The heavy chain of N3-1 is colored firebrick, and the light chain is colored coral. A lower resolution contour is shown to help visualize the Fabs and the RBDs. b Focused refinement map of N3-1 bound to RBDs in the up and down conformations. c One face of H-CDR3 contacts a conserved hydrophobic pocket (transparent royal blue surface) on RBD-up. The other face of H-CDR3 contacts the ACE2-binding site on RBD-down. H-CDR1 contacts the epitopes on RBD-up, but it is omitted for clarity. d Focused refinement map of N3-1 bound to RBD in the up conformation. e The epitope on RBD-down is centered on Phe486, which fits into a hydrophobic surface formed by Trp96, Tyr58, Tyr52, Arg100e and Val100g (clockwise). f Superimposed crystal structure of RBD-ACE2 complex (PDB ID: 6M0J) with N3-1 bound RBDs. The molecular surface of ACE2 is shown in transparent pale green. The light chain of N3-1 heavily clashes with ACE2. The ACE2-binding site on RBD-down is completely blocked by H-CDR2, H-CDR3 and L-CDR3 (e).
Understanding the Dynamics of SARS-CoV-2 Evolution
The global spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has exposed the vulnerability of our global health infrastructure to emerging pathogens. The rapid transmission of the virus, coupled with the absence of widespread adaptive immunity, has led to a pandemic that continues to strain healthcare systems worldwide. Previous variants, including Alpha, Beta, Gamma, and Delta, displayed increased transmissibility and infectiousness due to distinct spike protein mutations. However, the emergence of the Omicron variants has introduced unprecedented challenges, characterized by a multitude of mutations in crucial regions such as the N-terminal domain (NTD), receptor-binding domain (RBD), fusogenic stalk (S2), and furin cleavage site.
Omicron's Unique Immune Evasion Mechanism
Unlike earlier variants, Omicron's immune evasion cannot be solely attributed to amino acid substitutions disrupting antibody epitopes. Instead, conformational changes in the spike protein dynamics play
a pivotal role in evading conformationally biased antibodies. The Omicron lineage distinctly stabilizes a two-down, one-up (BA.1) or three-down (BA.2 and BA.5) state, altering the equilibrium of the receptor-binding domains (RBDs). This conformational shift poses a significant challenge for neutralizing antibodies, as observed in the study conducted at the University of Texas.
Innovative Antibody Discovery Strategies
To tackle the challenges posed by Omicron, the research team at the University of Texas adopted a multi-pronged antibody discovery and informatics strategy. This approach combined proteomic analysis of donor sera with the selection of combinatorial paired heavy and light chain (VH-VL) libraries. The goal was to identify potent neutralizing antibodies capable of effectively countering the evolving variants of SARS-CoV-2. Through this comprehensive strategy, the team identified a potent neutralizing antibody, N3-1, from a COVID-19 patient during the initial wave of the disease.
Structural Insights into N3-1 Binding Mode
The cryogenic electron microscopy (cryo-EM) structure of N3-1 proved to be of paramount importance, revealing a quaternary binding mode. In this mode, two N3-1 Fabs bind to a single trimeric spike, engaging RBDs in both the 'up' and 'down' conformations. This unique binding mode enhances the antibody's avidity and neutralization potency against major variants of concern, providing a promising avenue for therapeutic intervention. However, the study also illuminated the challenges posed by Omicron's altered RBD dynamics, particularly favoring the two-down, one-up state, which presents a significant obstacle to N3-1 neutralization.
Challenges Posed by Omicron BA.1 and Rational Design Solutions
The Omicron BA.1 variant, with its distinct set of mutations, presented a formidable challenge to N3-1 neutralization. Despite the application of structure-based rational design efforts, including amino acid substitutions in the antibody, full restoration of neutralization potency against BA.1 remained elusive. The study revealed that the altered dynamics of the RBDs in BA.1, particularly in the 371–376 loop, significantly contributed to immune escape.
Implications for Future Vaccine Design
The research findings have significant implications for future vaccine design strategies. Traditional vaccines often target specific epitopes on the virus, and the emergence of new variants can compromise their efficacy. The study suggests that future vaccine designs should consider incorporating stabilized constructs of alternate spike conformational states observed in Omicron. By exposing the immune system to a range of epitopes, including those presented in Omicron variants, vaccines may elicit a more broadly protective antibody response. This approach could potentially reduce susceptibility to common escape mutations and enhance overall vaccine efficacy.
Conclusion
In conclusion, the University of Texas study provides a comprehensive analysis of the immune evasion mechanisms employed by the Omicron variant. The dynamic interplay between viral evolution and the host immune response highlights the need for adaptive vaccine strategies that can anticipate and address conformational changes in the virus. This research serves as a crucial stepping stone toward a more comprehensive understanding of SARS-CoV-2 variants and informs future endeavors to enhance global immunity against the ever-evolving threat of COVID-19. As the scientific community continues to grapple with the challenges posed by emerging variants, ongoing research efforts remain essential for developing effective countermeasures and safeguarding public health worldwide.
The study findings were published in the peer reviewed journal: Communications Biology.
https://www.nature.com/articles/s42003-023-05649-6
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
