Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 12, 2025 6 months, 1 day, 23 hours, 47 minutes ago
Medical News: Carnitine Shuttle’s Surprising Role in Cancer
Scientists from the Translational‐Transdisciplinary Research Center at the Clinical Research Institute, Kyung Hee University Hospital at Gangdong, and the Department of Biomedical Science and Technology at Kyung Hee University, Republic of Korea, have uncovered a deeper link between the body’s fat‐burning machinery and cancer’s ability to resist treatment. Their latest review focuses on the “carnitine shuttle,” a system that moves fatty acids into mitochondria for energy production, and its surprising control over ferroptosis — an iron‐driven form of cell death that could be harnessed to destroy cancer cells.
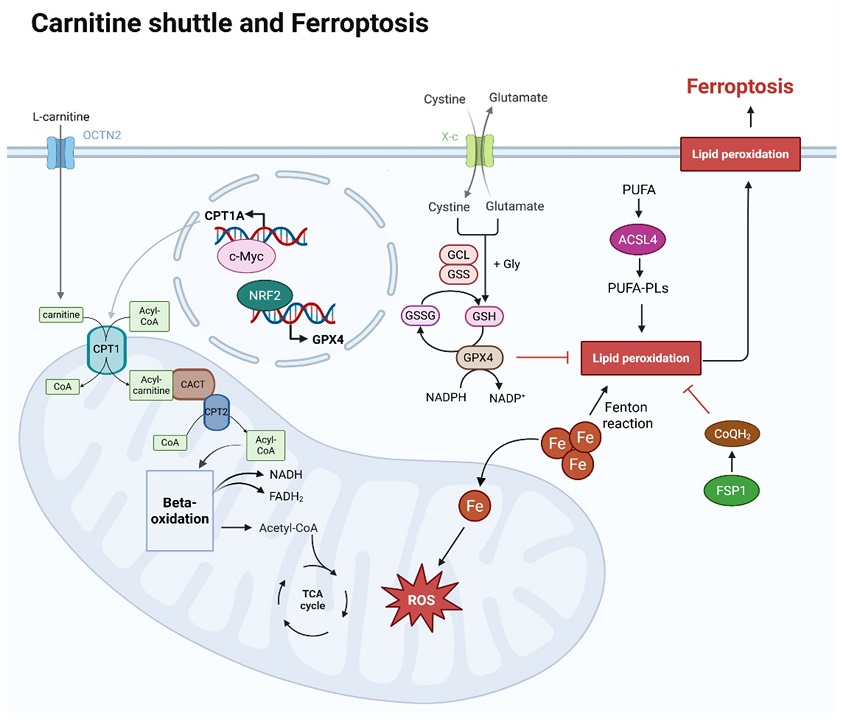 Overview of carnitine shuttle and ferroptosis within a cell. L-carnitine is transported by OCTN2 in the cell membrane, and the carnitine is transported into mitochondria and helps to transport CoA via the carnitine shuttle. Acyl-CoA, linked to long-chain fatty acids by the carnitine shuttle, enters the mitochondrial matrix, where long-chain fatty acids are used as an energy source in beta-oxidation. The acetyl-CoA produced at this stage then enters the TCA cycle, also generating NADH and FADH2. At this point, CPT1A expression is regulated by c-Myc. The mechanism of ferroptosis is iron-dependent. As iron flows into the mitochondria, it generates reactive oxygen species (ROS), which can lead to lipid peroxidation. Lipid peroxidation susceptibility increases as polyunsaturated fatty acids (PUFAs) are oxidized and converted into phospholipid (PUFA-PLs) forms. This process is promoted by ACSL4. However, GPX4 and CoQH2 can inhibit lipid peroxidation. After cystine uptake and conversion to cysteine, GSH is produced, and both GSH and GPX4 inhibit lipid peroxidation. The expression of GPX4 is regulated by NRF2. CoQH2, converted from CoQ by FSP1, also inhibits lipid peroxidation
Overview of carnitine shuttle and ferroptosis within a cell. L-carnitine is transported by OCTN2 in the cell membrane, and the carnitine is transported into mitochondria and helps to transport CoA via the carnitine shuttle. Acyl-CoA, linked to long-chain fatty acids by the carnitine shuttle, enters the mitochondrial matrix, where long-chain fatty acids are used as an energy source in beta-oxidation. The acetyl-CoA produced at this stage then enters the TCA cycle, also generating NADH and FADH2. At this point, CPT1A expression is regulated by c-Myc. The mechanism of ferroptosis is iron-dependent. As iron flows into the mitochondria, it generates reactive oxygen species (ROS), which can lead to lipid peroxidation. Lipid peroxidation susceptibility increases as polyunsaturated fatty acids (PUFAs) are oxidized and converted into phospholipid (PUFA-PLs) forms. This process is promoted by ACSL4. However, GPX4 and CoQH2 can inhibit lipid peroxidation. After cystine uptake and conversion to cysteine, GSH is produced, and both GSH and GPX4 inhibit lipid peroxidation. The expression of GPX4 is regulated by NRF2. CoQH2, converted from CoQ by FSP1, also inhibits lipid peroxidation
Ferroptosis is unlike other forms of cell death such as apoptosis. It happens when iron triggers harmful oxidative damage to the fats in cell membranes, eventually leading to the cell’s collapse. This
Medical News report reveals how cancer cells manipulate the carnitine shuttle, especially through a key enzyme called CPT1A, to block ferroptosis and survive even harsh treatments like chemotherapy, radiotherapy, and immunotherapy.
How Cancer Cells Exploit the Carnitine Shuttle
The carnitine shuttle is made up of CPT1A, CPT2, and CACT. CPT1A sits on the outer mitochondrial membrane and decides how much fat can enter for energy production. Many aggressive cancers, including breast, colorectal, lung, and prostate cancers, show unusually high CPT1A activity. This boosts fatty acid oxidation (FAO), providing cancer cells with energy and antioxidant power to fight off ferroptosis.
A key discovery is that tumor‐associated macrophages (TAMs) in the cancer microenvironment produce L‐carnitine, a molecule essential for this shuttle system. Cancer stem cells then absorb this L‐carnitine through a transporter called OCTN2, making them even more resistant to ferroptosis. This nutrient sharing between cancer cells and surrounding immune cells creates a metaboli
c shield that protects tumors from treatment.
The CPT1A and c‐Myc Feedback Loop
Researchers also identified a self‐reinforcing loop between CPT1A and the cancer‐promoting gene c‐Myc. CPT1A helps stabilize c‐Myc, while c‐Myc boosts CPT1A production. This loop strengthens cancer cells’ antioxidant defenses, reduces the fats that ferroptosis targets, and makes them harder to kill.
Therapeutic Opportunities
Targeting the carnitine shuttle opens a new door in cancer therapy. Experimental drugs such as etomoxir, perhexiline, and mildronate aim to block CPT1A or L‐carnitine supply, making cancer cells more vulnerable to ferroptosis. Combining these drugs with immunotherapy has shown promise in preclinical studies by boosting immune cell infiltration and increasing tumor death.
Conclusion
This new understanding positions the carnitine shuttle not just as a metabolic pathway but as a powerful survival tool for cancer cells. Disrupting it could strip tumors of their energy reserves, weaken their antioxidant shield, and make them more sensitive to ferroptosis and other treatments. While challenges remain in drug safety, patient selection, and overcoming resistance, the findings point to a promising strategy for tackling treatment‐resistant cancers and improving patient outcomes.
The study findings were published in the peer reviewed journal: Antioxidants.
https://www.mdpi.com/2076-3921/14/8/972
For the latest cancer news, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/tropisetron-found-to-prevent-pancreatic-cancer-by-blocking-dangerous-inflammation-trigger
https://www.thailandmedical.news/news/breaking-medical-researchers-warn-that-covid-19-promotes-development-of-testicular-tumors
https://www.thailandmedical.news/news/breaking-covid-19-infection-triggers-genetic-change-in-thyroid-tissues-that-accelerates-thyroid-cancer-progression
https://www.thailandmedical.news/articles/cancer
