Study Finds That Proximal Immune-Epithelial Progenitor Interactions Drive Chronic Tissue Sequelae Post COVID-19
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 19, 2023 2 years, 5 months, 5 days, 20 hours, 34 minutes ago
COVID-19 News: The COVID-19 pandemic has had a profound impact on global health, with millions of people worldwide affected by the acute respiratory illness caused by the SARS-CoV-2 virus. While significant strides have been made in treating acute COVID-19 and developing vaccines, a growing concern has emerged regarding the long-term health effects of the disease. This condition, often referred to as Post-Acute Sequelae of COVID-19 (PASC) or Long COVID, presents a complex array of symptoms and complications that persist long after the acute infection has resolved. Among the various manifestations of PASC, respiratory sequelae pose a particularly challenging problem, with some individuals developing a condition known as PASC pulmonary fibrosis (PASC-PF).
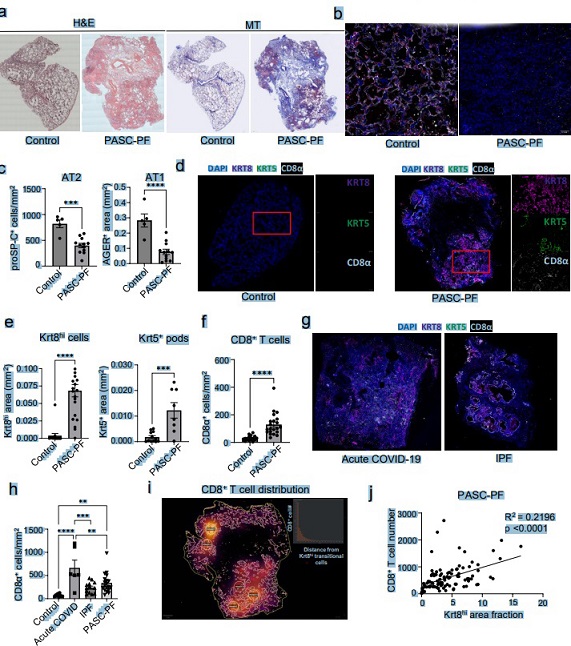 Lung histology was notable for extensive immune cell infiltration and collagen deposition in the alveolar epithelium (a). Consistent with the observed fibrotic sequelae, we found reduced levels of AT1 and AT2 cells in PASC-PF lungs compared to controls, suggesting a persistent defect in alveolar regeneration (b,c). We also bserved chronic persistence of ectopic Krt5+ basal cells and Krt8hi transitional cells in PASC-PF ungs compared to controls, which is concordant with recent reports (d,e). PASC-PF lungs also harbored widespread expression of alpha smooth muscle actin (αSMA), indicative of myofibroblast activity in addition to pockets of Krt5- Krt17+ aberrant basaloid cells previously found in IPF lungs (a). Therefore, PASC-PF is characterized by the sustained loss of functional alveolar epithelial cells, and the persistence of dysplastic Krt5+ pods and Krt8hi transitional cells, which is histologically akin to other fibrotic lung diseases such as idiopathic pulmonary fibrosis (IPF). Previously, we reported that increased CD8+ T cell levels in the bronchoalveolar lavage (BAL) fluid were associated with impaired lung function of COVID-19 convalescents. In accordance, CD8+ T cell numbers were elevated in PASC-PF patient lungs (f). Moreover, CD8+ T cell abundance was significantly higher in acute COVID-19 but not in IPF lungs when compared to controls (g,h). Interestingly, a striking spatial association was observed between CD8+ T cells, and Krt8hi and Krt5+ areas representing dysplastic repair upon analysis of their distribution in PASC-PF lungs (i,j, b,c,h). Notably, this correlation between CD8+ T cells, and Krt8hi and Krt5+ dysplastic areas was unique to PASC-PF lungs but not seen in lungs from control, acute COVID-19 or IPF conditions (d-g,i,j). Collectively, these data indicate that the spatiotemporal colocalization of CD8+ T cells and areas of dysplastic repair is a unique feature of post-viral pulmonary fibrosis and supports immune-epithelial progenitor interactions potentially contributing to the observed defects in alveolar regeneration and chronic pulmonary sequelae.
Lung histology was notable for extensive immune cell infiltration and collagen deposition in the alveolar epithelium (a). Consistent with the observed fibrotic sequelae, we found reduced levels of AT1 and AT2 cells in PASC-PF lungs compared to controls, suggesting a persistent defect in alveolar regeneration (b,c). We also bserved chronic persistence of ectopic Krt5+ basal cells and Krt8hi transitional cells in PASC-PF ungs compared to controls, which is concordant with recent reports (d,e). PASC-PF lungs also harbored widespread expression of alpha smooth muscle actin (αSMA), indicative of myofibroblast activity in addition to pockets of Krt5- Krt17+ aberrant basaloid cells previously found in IPF lungs (a). Therefore, PASC-PF is characterized by the sustained loss of functional alveolar epithelial cells, and the persistence of dysplastic Krt5+ pods and Krt8hi transitional cells, which is histologically akin to other fibrotic lung diseases such as idiopathic pulmonary fibrosis (IPF). Previously, we reported that increased CD8+ T cell levels in the bronchoalveolar lavage (BAL) fluid were associated with impaired lung function of COVID-19 convalescents. In accordance, CD8+ T cell numbers were elevated in PASC-PF patient lungs (f). Moreover, CD8+ T cell abundance was significantly higher in acute COVID-19 but not in IPF lungs when compared to controls (g,h). Interestingly, a striking spatial association was observed between CD8+ T cells, and Krt8hi and Krt5+ areas representing dysplastic repair upon analysis of their distribution in PASC-PF lungs (i,j, b,c,h). Notably, this correlation between CD8+ T cells, and Krt8hi and Krt5+ dysplastic areas was unique to PASC-PF lungs but not seen in lungs from control, acute COVID-19 or IPF conditions (d-g,i,j). Collectively, these data indicate that the spatiotemporal colocalization of CD8+ T cells and areas of dysplastic repair is a unique feature of post-viral pulmonary fibrosis and supports immune-epithelial progenitor interactions potentially contributing to the observed defects in alveolar regeneration and chronic pulmonary sequelae.
A new study conducted by researchers from the University of Virginia in the USA, Cedars-Sinai Medical Center in the USA, Mayo Clinic in the USA, and Chung-Ang University in South Korea sheds light on the underlying cellular and molecular mechanisms driving chronic tissue sequelae in respiratory PASC and identifies potential therapeutic targets for mitigating these sequelae.
Understanding PASC and PASC-PF
PASC is characterized by persistent and often debilitating symptoms that affect various organ systems, in
cluding the respiratory system as covered in various past studies and
COVID-19 News reports. Individuals with PASC may experience dyspnea, compromised lung function, radiological abnormalities, and a range of extrapulmonary symptoms. However, one of the most concerning manifestations of respiratory PASC is the development of PASC-PF, a condition that results in fibrotic scarring of the lung tissue. Patients with PASC-PF often require long-term oxygen supplementation and, in severe cases, lung transplantation.
The Mechanisms Underlying PASC-PF
Despite the increasing recognition of PASC and PASC-PF, the exact mechanisms that drive these chronic tissue sequelae remain poorly understood. Researchers have observed common histopathological features in the lungs of PASC-PF patients, such as the persistent reduction in alveolar epithelial cells and the presence of dysplastic progenitors expressing cytokeratin 8 (Krt8) and Krt5.
Additionally, various immune cell populations continue to inhabit the lungs of PASC-PF patients. However, the interactions between immune cells and epithelial progenitors in the context of post-viral fibrosis have not been thoroughly explored until now.
The Need for Clinically Relevant Animal Models
To gain insights into the mechanisms driving PASC-PF, it is essential to establish clinically relevant animal models that recapitulate the pathological and immunological features observed in human patients. While several animal models have been developed to study acute COVID-19, they do not fully capture the complexities of PASC-PF. For instance, studies using SARS-CoV-2-infected mice have not reproduced the persistent Krt8hi and Krt5+ areas characteristic of human PASC-PF. Furthermore, the role of CD8+ T cells, which are enriched in PASC-PF lungs, in promoting the maintenance of dysplastic areas has not been adequately explored in these models.
A Model for Studying PASC-PF: Aged Influenza-Infected Mice
In this groundbreaking study, researchers turned to an alternative approach by using aged C57BL/6 mice infected with influenza virus. Surprisingly, this mouse model exhibited chronic pulmonary sequelae that closely mirrored the immunopathological features observed in human PASC-PF lungs. Notably, the infiltration and accumulation of profibrotic monocyte-derived macrophages were evident in both human PASC-PF and the mouse model. This discovery suggests that CD8+ T cells, which play a pivotal role in impaired recovery and fibrotic remodeling in PASC-PF, contribute to the development of fibrotic disease.
The Role of CD8+ T Cells in PASC-PF
In healthy individuals, CD8+ T cells are mobilized to protect vulnerable lung sites in case of reinfection, forming repair-associated memory depots. In acute COVID-19 cases with successful recovery, pulmonary CD8+ T cells gradually decline as alveolar regeneration takes place. However, in PASC-PF patients and the aged influenza-infected mouse model, these CD8+ T cells persist in the lungs, hindering lung recovery and promoting fibrotic disease. It is yet to be determined whether CD8+ T cells also contribute to the development of idiopathic pulmonary fibrosis (IPF) or if they primarily affect the balance between functional recovery and PASC-PF.
The Role of Interferon-Gamma (IFN-γ), Tumor Necrosis Factor (TNF), and Interleukin-1β (IL-1β)
To elucidate the mechanisms underlying respiratory sequelae post-viral infections, the researchers employed imaging and spatial transcriptomics techniques. Their findings indicate that chronic IL-1β signaling, mediated by IFN-γ and TNF, plays a central role in driving the pathogenesis of PASC-PF. While in vitro experiments demonstrated that chronic IL-1β impairs the trans-differentiation of alveolar epithelial cells, further research is needed to investigate its impact on Krt8hi and Krt5+ progenitors in vivo. Importantly, the study revealed that neutralizing IFN-γ and TNF, or IL-1β, after the resolution of acute infection significantly improved alveolar regeneration and mitigated fibrotic sequelae.
Therapeutic Implications
The research findings hold significant promise for the development of therapeutic strategies to address PASC-PF and related respiratory sequelae in COVID-19 patients. Notably, drugs like the IL-1 receptor antagonist, Anakinra, and the JAK inhibitor, Baricitinib, have already received emergency use authorization from the United States Food and Drug Administration for the treatment of acute COVID-19. Given their potential to dampen chronic pulmonary sequelae, these drugs may prove to be valuable candidates for managing ongoing respiratory PASC.
Conclusion
As the world continues to grapple with the ongoing challenges posed by the COVID-19 pandemic, understanding and effectively managing the long-term consequences of the disease is of paramount importance. This comprehensive study conducted by a collaborative team of researchers from esteemed institutions sheds light on the intricate mechanisms driving chronic tissue sequelae in respiratory PASC, particularly PASC-PF. By using a clinically relevant animal model and advanced techniques, they have identified the crucial role of CD8+ T cells and proinflammatory cytokines in promoting fibrotic remodeling. The findings not only enhance our understanding of PASC-PF but also provide a foundation for the development of targeted therapies that may improve the quality of life for individuals grappling with these debilitating long-term effects of COVID-19.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2023.09.13.557622v1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
