BREAKING NEWS! Study Discovers That RAGE Is A Receptor For SARS-CoV-2 N Protein And Mediates N Protein-Induced Acute Lung Injury
Thailand Medical News Team Aug 05, 2023 2 years, 6 months, 5 days, 17 hours, 14 minutes ago
COVID-19 News: In a groundbreaking study conducted at Zhejiang University School of Medicine Children's Hospital in China, researchers have made a significant discovery in the fight against COVID-19. The study found that the receptor for advanced glycation endproducts (RAGE) plays a crucial role in mediating acute lung injury induced by the nucleocapsid protein (N protein) of the SARS-CoV-2 virus. This study finding sheds new light on the mechanism of lung inflammation in COVID-19 and offers potential therapeutic targets to combat the disease.
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has wreaked havoc worldwide, leading to unprecedented public health and socioeconomic consequences as covered in numerous past
COVID-19 News reports. Among the severe complications of the disease, acute lung injury and acute respiratory distress syndrome (ARDS) are the most serious and frequent, contributing to a high mortality rate. Previous studies have shown that the N protein of the virus is directly involved in lung inflammation and injury. However, the specific signaling mechanism that triggers the inflammatory response remained unknown.
To address this knowledge gap, the study team set out to investigate whether RAGE, a transmembrane glycoprotein with high expression in lung tissues, could be the missing link between the N protein and acute lung injury. They performed protein-protein interaction assays and found a direct binding between the N protein and RAGE. Further investigations using various techniques, such as flow cytometry-based binding assay, surface plasmon resonance, and enzyme-linked immunosorbent assay, confirmed this binding.
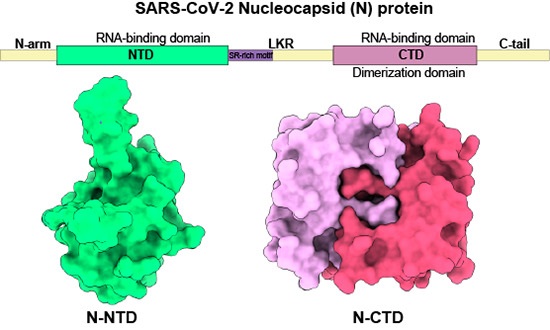
Remarkably, the study revealed that N protein binds to RAGE via both its N-terminal and C-terminal domains. This finding implies that multiple interactions between N protein and RAGE may strengthen the activation of the RAGE-ERK1/2-NF-ĸB signaling pathway, which plays a pivotal role in promoting the proinflammatory response in the lung.
To validate the significance of RAGE in N protein-induced acute lung injury, the study team conducted in vitro and in vivo experiments. They treated cells with recombinant N protein and observed the activation of the RAGE-ERK1/2-NF-ĸB pathway, leading to a proinflammatory response. Conversely, RAGE-deficient cells displayed reduced proinflammatory signaling and response upon N protein stimulation.
The study team also studied N protein-induced acute lung injury in mice by employing RAGE deficiency and small molecule antagonists. The results were striking, as RAGE deficiency and RAGE inhibition partially protected the mice from N protein-induced lung injury. This crucial evidence establishes RAGE as a significant player in the development of acute lung injury in COVID-19.
The discovery of RAGE as a receptor for the N protein not only deepens our understanding of the pathogenesis of COVID-19 but also opens up new avenues for potential therapeutic interventions. The study suggests that targeting RAGE could help mitigate the cytokine storm and ARDS, which are major contributors to COVID-19 mortality.
Moreover, this study adds to the growing body of research on the role of RAGE in COVID-19. Prior inve
stigations have identified RAGE as a biomarker for predicting disease severity and the need for mechanical ventilation. Additionally, RAGE antagonist treatments have shown promising results in improving survival and reducing lung inflammation in SARS-CoV-2 infected mice.
Although this study makes significant strides in unravelling the relationship between the N protein and RAGE in COVID-19-induced lung injury, there are still some questions to be addressed. For instance, while RAGE deficiency and inhibition partially protected against N protein-induced lung injury, there might be other binding partners of N protein that contribute to the overall pathogenesis.
Nevertheless, this breakthrough research offers valuable insights into the complex molecular mechanisms underlying COVID-19 and highlights RAGE as a potential therapeutic target for managing the disease. The search for effective treatments for COVID-19 continues, and with this newfound understanding of the N protein-RAGE interaction, scientists and medical professionals are one step closer to developing targeted therapies to combat this global pandemic.
The key findings of the study were:
-RAGE is a receptor for N-protein
-N-protein activates RAGE-ERK1/2-NF-ĸB pathway
-N-NTD and N-CTD mimic full-length N-protein in signaling and inflammatory response
-RAGE deficiency reduces N-protein-induced ERK1/2-NF-ĸB signaling and proinflammatory response
-RAGE deficiency and inhibition alleviate N-protein-induced acute lung injury
The study findings were published in the peer reviewed American Journal for Respiratory Cell and Molecular Biology.
https://www.atsjournals.org/doi/abs/10.1165/rcmb.2022-0351OC
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
More About RAGE
RAGE (receptor for advanced glycation endproducts), also called AGER, is a 35 kilodalton transmembrane receptor of the immunoglobulin super family Its name comes from its ability to bind advanced glycation endproducts (AGE), which include chiefly glycoproteins, the glycans of which have been modified non-enzymatically through the Maillard reaction. In view of its inflammatory function in innate immunity and its ability to detect a class of ligands through a common structural motif, RAGE is often referred to as a pattern recognition receptor. RAGE also has at least one other agonistic ligand: high mobility group protein B1 (HMGB1). HMGB1 is an intracellular DNA-binding protein important in chromatin remodeling which can be released by necrotic cells passively, and by active secretion from macrophages, natural killer cells, and dendritic cells.
The interaction between RAGE and its ligands is thought to result in pro-inflammatory gene activation. Due to an enhanced level of RAGE ligands in diabetes or other chronic disorders, this receptor is hypothesized to have a causative effect in a range of inflammatory diseases such as diabetic complications, Alzheimer's disease and even some tumors.
Isoforms of the RAGE protein, which lack the transmembrane and the signaling domain (commonly referred to as soluble RAGE or sRAGE) are hypothesized to counteract the detrimental action of the full-length receptor and are hoped to provide a means to develop a cure against RAGE-associated diseases.
The RAGE gene lies within the major histocompatibility complex (MHC class III region) on chromosome 6 and comprises 11 exons interlaced by 10 introns. Total length of the gene is about 1400 base pairs (bp) including the promoter region, which partly overlaps with the PBX2 gene. About 30 polymorphisms are known most of which are single-nucleotide polymorphisms.
RAGE has been linked to several chronic diseases, which are thought to result from vascular damage. The pathogenesis is hypothesized to include ligand binding, upon which RAGE signals activation of nuclear factor kappa B (NF-κB). NF-κB controls several genes involved in inflammation. RAGE itself is upregulated by NF-κB. Given a condition in which there is a large amount of RAGE ligands (e.g. AGE in diabetes or amyloid-β-protein in Alzheimer's disease) this establishes a positive feed-back cycle, which leads to chronic inflammation. This chronic condition is then believed to alter the micro- and macrovasculature, resulting in organ damage or even organ failure.
However, whilst RAGE is up-regulated in inflammatory conditions, it is down-regulated in lung cancer and pulmonary fibrosis. Diseases that have been linked to RAGE are:
-Alzheimer's disease
-Arthritis
-Atherosclerosis
-Congestive heart failure
-Congenital diaphragmatic hernia
-Diabetes
-Myocardial infarction
-Peripheral vascular disease
-Psoriasis
-Rheumatoid arthritis
-Takayasu's arteritis
-Schizophrenia
RAGE is expressed at the highest levels in the lung compared to other tissues, in particular in alveolar type I cells, and is lost in idiopathic pulmonary fibrosis (IPF) indicating that expression and regulation of RAGE in the pulmonary system differs from that in the vascular system. Blockade/knockdown of RAGE resulted in impaired cell adhesion, and increased cell proliferation and migration
A number of small molecule RAGE inhibitors or antagonists have been reported including the drug: Azeliragon
