BREAKING! Boston University Discovers That Vascular Protein Vimentin Assists SARS-CoV-2 Access Into Cells, Contributing To Vascular Complications!
Source: SARS-CoV-2 Research Jan 27, 2022 4 years, 4 weeks, 1 day, 25 minutes ago
SARS-CoV-2 Research: A new study by researchers from Boston University School of Medicine has found that the human host vascular protein called Vimentin actually assists the SARS-CoV-2 coronavirus get access into cells and such actions can also contribute to vascular complications!
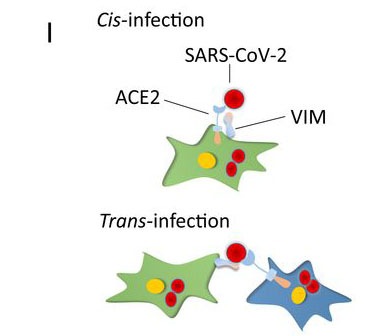
To date, the human angiotensin-converting enzyme 2 (ACE2) is the most widely known entry receptor for SARS-CoV-2 although the virus can also use a wide range of other identified receptors as well such as CD147, Neuropilin‐1, Dipeptidyl peptidase 4, alanyl aminopeptidase (ANPEP), glutamyl aminopeptidase (ENPEP) and angiotensin II receptor type 2 (AGTR2).
However, the possible involvement of other cellular components in viral entry mechanisms remains unknown.
The
SARS-CoV-2 Research found that Vimentin is expressed in human endothelial cells, binds to SARS-CoV-2-spike, and expedites SARS-CoV-2 entry. Treatment of lung ACE2/A549 carcinoma cells with purified vimentin or coculture of ACE2/A549 cells with HEK-293 cells expressing vimentin increased ACE2-dependent viral entry. CR3022 antibody blocked vimentin interaction with SARS-CoV-2-spike and inhibited SARS-CoV-2 entry.
The study found that Vimentin could facilitate SARS-CoV-2 infection and contribute to vascular complications associated with COVID-19.
The study findings describe the identification of vimentin (VIM), an intermediate filament protein widely expressed in cells of mesenchymal origin, as an important attachment factor for SARS-CoV-2 on human endothelial cells.
Utilizing liquid chromatography–tandem mass spectrometry, the study team identified VIM as a protein that binds to the SARS-CoV-2 spike (S) protein.
The study team showed that the S-protein receptor binding domain (RBD) is sufficient for S-protein interaction with VIM. Further analysis revealed that extracellular VIM binds to SARS-CoV-2 S-protein and facilitates SARS-CoV-2 infection, as determined by entry assays performed with pseudotyped viruses expressing S and with infectious SARS-CoV-2. Coexpression of VIM with ACE2 increased SARS-CoV-2 entry in HEK-293 cells, and shRNA-mediated knockdown of VIM significantly reduced SARS-CoV-2 infection of human endothelial cells.
Furthermore, incubation of A549 cells expressing ACE2 with purified VIM increased pseudotyped SARS-CoV-2-S entry. CR3022 antibody, which recognizes a distinct epitope on SARS-CoV-2-S-RBD without interfering with the binding of the spike with ACE2, inhibited the binding of VIM with CoV-2 S-RBD, and neutralized viral entry in human endothelial cells, suggesting a key role for VIM in SARS-CoV-2 infection of endothelial cells.
The study findings provide insight into the pathogenesis of COVID-19 linked to the vascular system, with implications for the development of therapeutics and vaccines.
The study findings were published in the peer reviewed journal: PNAS (Proceedings of the National Academy of Sciences of the United States of America)
https://www.pnas.org/content/119/6/e2113874119
The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells is
an essential step for virus transmission, virus tropism and pathogenesis.
Though the lung epithelial cells are its initial target, SARS-CoV-2 also can infect endothelial cells. Endothelial cells are the major constituents of the vascular system and cardiovascular complication is a hallmark of severe COVID-19. Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV-2.
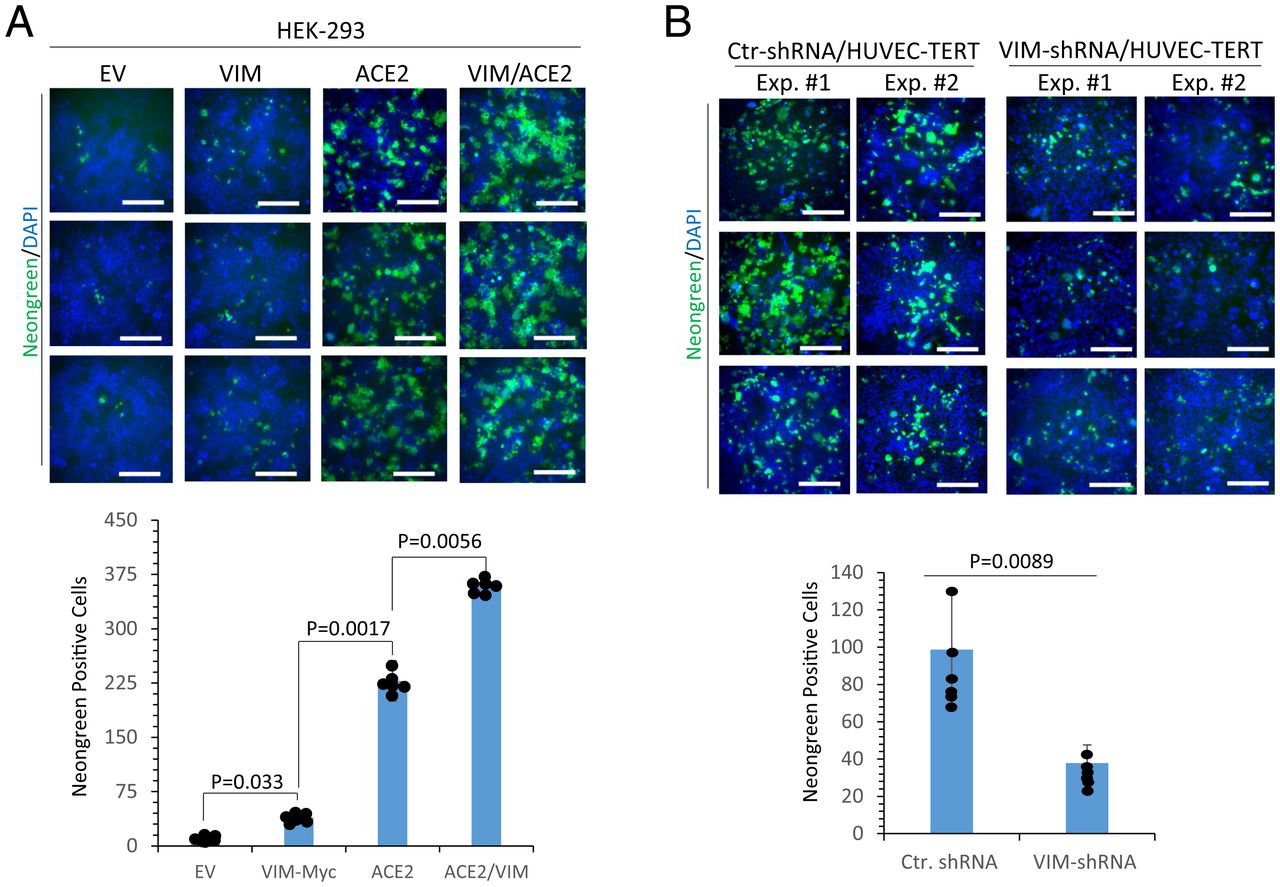 VIM is an attachment factor for SARS-CoV-2 and enhances ACE2-dependent viral entry. (A) HEK-293 cells expressing EV, VIM-Myc, ACE2, or coexpressing VIM-Myc with ACE2 were seeded in 96-well plates (triplicate per group). The next day, the cells were infected with SARS-CoV-2-mNG at an MOI of 2. Twenty-four hours postinfection, the cells were fixed, stained with DAPI, and analyzed by fluorescence microscopy. Three pictures per condition were taken from random fields. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (B) HUVEC-TERT cells expressing control shRNA or VIM-shRNA were infected with SARS-CoV-2-mNG as described for A. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (Scale bars, 50 µm.)
VIM is an attachment factor for SARS-CoV-2 and enhances ACE2-dependent viral entry. (A) HEK-293 cells expressing EV, VIM-Myc, ACE2, or coexpressing VIM-Myc with ACE2 were seeded in 96-well plates (triplicate per group). The next day, the cells were infected with SARS-CoV-2-mNG at an MOI of 2. Twenty-four hours postinfection, the cells were fixed, stained with DAPI, and analyzed by fluorescence microscopy. Three pictures per condition were taken from random fields. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (B) HUVEC-TERT cells expressing control shRNA or VIM-shRNA were infected with SARS-CoV-2-mNG as described for A. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (Scale bars, 50 µm.)
Until this study, the possible involvement of other cellular components in the viral entry was not fully understood.
The study team from Boston University School of Medicine (BUSM) has identified extracellular vimentin as an attachment factor that facilitates SARS-CoV-2 entry into human cells. Vimentin is a structural protein that is widely expressed in the cells of mesenchymal origin such as endothelial cells and a potential novel target against SARS-CoV-2, which could block the infection of the SARS-CoV-2.
Corresponding author Dr Nader Rahimi, Ph.D., associate professor of pathology & laboratory medicine at BUSM told Thailand
Medical News, "Severe endothelial injury, vascular thrombosis, and obstruction of alveolar capillaries (tiny air sacs scattered throughout the lungs) are common features of severe COVID-19. Identification of vimentin as a host attachment factor for SARS-CoV-2 can provide new insight into the mechanism of SARS-CoV-2 infection of the vascular system and can lead to the development of novel treatment strategies.”
The study team used liquid chromatography–tandem mass spectrometry (LC-MS/MS) and identified vimentin as a protein that binds to the SARS-CoV-2 spike (S) protein and facilitates SARS-CoV-2 infection. They also found that depletion of vimentin significantly reduces SARS-CoV-2 infection of human endothelial cells. In contrast, over-expression of vimentin with ACE2 significantly increased the infection rate.
Dr Rahimi explained, "More importantly, we saw that the CR3022 antibody inhibited the binding of vimentin with CoV-2-S-protein, and neutralized SARS-CoV-2 entry into human cells."
The study team concluded, “Vimentin is widely expressed in human cells with mesenchymal origin, including endothelial cells, fibroblasts, macrophages, melanocytes, and lymphocytes. Additionally, Vimentin is expressed in alveolar type 2 cells and nasal goblet secretory cells, which are also positive for ACE2, and its expression is up-regulated in response to viral infection and inflammatory stimuli, suggesting a potential role for Vimentin in infection of nasal and lung epithelial cells. In addition to its participation in infection, Vimentin also plays a critical role in lung inflammation and fibrosis through interaction with NLRP3 (NACHT, LRR, and PYD domains-containing protein 3). Expression of ACE2 in endothelial cells and lung alveolar cells is relatively low, but Vimentin as an attachment factor produced by mesenchymal cells (e.g., endothelial and fibroblasts), could enhance the SARS-CoV-2 entry in respiratory cells via both cis- and trans-infection. Consistent with this idea, coculture of Vimentin/HEK-293 cells with ACE2/A549 cells increased viral entry. Taken together, the observations we present may lead to the development of new antiviral therapeutics combining therapies that inhibit interactions of both ACE2 and VIM with SARS-CoV-2.”
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.

 VIM is an attachment factor for SARS-CoV-2 and enhances ACE2-dependent viral entry. (A) HEK-293 cells expressing EV, VIM-Myc, ACE2, or coexpressing VIM-Myc with ACE2 were seeded in 96-well plates (triplicate per group). The next day, the cells were infected with SARS-CoV-2-mNG at an MOI of 2. Twenty-four hours postinfection, the cells were fixed, stained with DAPI, and analyzed by fluorescence microscopy. Three pictures per condition were taken from random fields. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (B) HUVEC-TERT cells expressing control shRNA or VIM-shRNA were infected with SARS-CoV-2-mNG as described for A. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (Scale bars, 50 µm.)
VIM is an attachment factor for SARS-CoV-2 and enhances ACE2-dependent viral entry. (A) HEK-293 cells expressing EV, VIM-Myc, ACE2, or coexpressing VIM-Myc with ACE2 were seeded in 96-well plates (triplicate per group). The next day, the cells were infected with SARS-CoV-2-mNG at an MOI of 2. Twenty-four hours postinfection, the cells were fixed, stained with DAPI, and analyzed by fluorescence microscopy. Three pictures per condition were taken from random fields. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (B) HUVEC-TERT cells expressing control shRNA or VIM-shRNA were infected with SARS-CoV-2-mNG as described for A. Graph is representative of SARS-CoV-2-mNG+ cells (triplicate well per group, two independent experiments). (Scale bars, 50 µm.)