Red Blood Cell Fragments and Dying Blood Vessels May Be the Real Cause of COVID-19 Related Organ Damage
Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 15, 2025 8 months, 1 week, 1 day, 21 hours, 57 minutes ago
Thailand Medical News: A Radical Rethink of COVID Blood Vessel Blockages
In a groundbreaking discovery that could dramatically shift how we understand severe COVID-19 complications, researchers from the University of Sydney, in collaboration with global institutions, have uncovered a surprising culprit behind blood vessel blockages in COVID-19 patients—not the usual blood clots formed by platelets and fibrin, but rather ruptured red blood cells accumulating at dying blood vessel walls.
 Red Blood Cell Fragments and Dying Blood Vessels May Be the Real Cause of COVID-19 Related Organ Damage
Red Blood Cell Fragments and Dying Blood Vessels May Be the Real Cause of COVID-19 Related Organ Damage
This unexpected finding challenges long-held beliefs about how the virus damages the body and why traditional treatments like anticoagulants often fall short. Instead of classical thrombosis being the main cause of microvascular obstruction, the researchers found that red blood cell rupture, known as hemolysis, caused by dying endothelial cells (the inner lining of blood vessels), may be a more significant factor. This
Thailand Medical News report unpacks the study in detail and explains its potentially life-saving implications.
Why Microvascular Injury Matters in COVID-19
Severe COVID-19 is frequently associated with damage to the smallest blood vessels—called capillaries—in vital organs like the lungs, heart, kidneys and liver. These microvascular injuries have been blamed for sudden organ failure in some patients and long-lasting symptoms in others. Until now, the prevailing belief was that this damage came from microclots—tiny blood clots formed by fibrin and platelets.
But despite widespread use of blood-thinning medications to prevent these clots, many patients still suffered from persistent vessel blockage and related complications. This inconsistency led scientists to question whether something else might be at play. The research team, led by experts from the Charles Perkins Centre and School of Medical Sciences at the University of Sydney, along with partners from Royal Prince Alfred Hospital and other institutions, sought to find out.
What the Researchers Did
The researchers conducted a meticulous investigation using over 1,000 autopsy samples from COVID-19 patients, focusing on tissue from the lungs, heart, kidneys and liver. Importantly, they excluded any samples that showed signs of post-mortem tissue decay to ensure accuracy.
Using advanced imaging techniques and histological analysis, they examined the state of the blood vessels in these organs. They looked for key markers that would indicate the presence of clots, such as fibrin and platelets, as well as signs of red blood cell damage.
A Shocking Discovery
Instead of finding typical clotting markers, the researchers found widespread red blood cell membrane fragments deposited along the walls of blood vessels, especially in the heart, liver and kidneys. These fragments stained strongly for CD235, a marker specific to red blood cells, but not for fibrin or
platelets.
Even more striking was the loss of structural integrity in the endothelial cells—often detached or completely missing—indicating extensive cell death. In some organs, up to 50 percent of the small vessels examined showed signs of this endothelial damage.
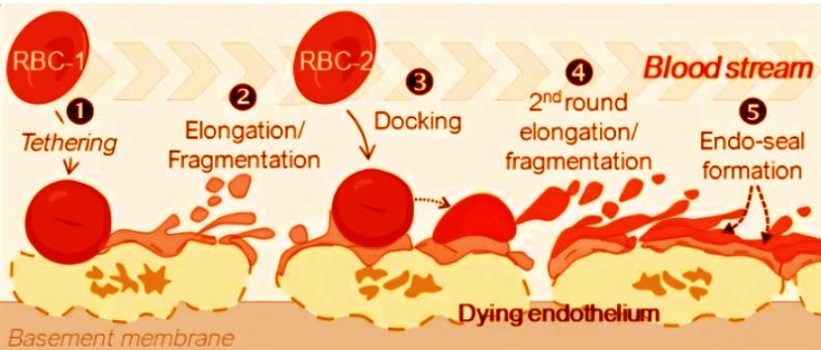
The membrane fragments from ruptured red blood cells had accumulated into sticky masses that physically blocked the flow of blood, even without the involvement of platelets or fibrin. This suggests a completely different mechanism of blockage: one driven by cellular debris, not coagulation.
The Role of Necroptosis and Complement
To understand why this was happening, the team turned to mouse models and in vitro systems. They found that ischemia—a condition where blood supply is temporarily cut off and then restored—triggers a special kind of cell death called necroptosis in endothelial cells. This form of cell death is orchestrated by specific proteins, including MLKL and RIPK3.
As these endothelial cells die, they activate the complement system, a part of the immune system that helps destroy pathogens. However, this activation also leads to red blood cell lysis, causing the cells to rupture and release their membranes.
The researchers genetically modified mice to lack the MLKL protein in endothelial cells, which drastically reduced both red blood cell destruction and vessel blockage. Similarly, mice that lacked complement protein C9 also showed less red blood cell damage, confirming that both pathways are critical for this process.
Real-Time Proof of a New Mechanism
The team used intravital microscopy—a live imaging technique—to visualize how the debris from lysed red cells interacts with the damaged endothelium. They observed that red blood cell membranes clung to necrotic vessel walls, forming physical barriers that interrupted blood flow. This happened independently of traditional clotting agents like platelets or fibrin.
Interestingly, in mice lacking the necroptosis or complement pathways, there was more bleeding in the microvessels, implying that while the red cell fragments can block flow, they also serve a hemostatic role—helping to stop internal bleeding in the absence of clotting factors.
Implications for COVID-19 and Beyond
These findings have profound implications. They explain why many COVID-19 patients still suffer from blood vessel obstruction even when treated with anticoagulants—because the issue isn't a clot in the classical sense. It's a build-up of red blood cell debris triggered by dying blood vessel walls.
The same red cell rupture pattern was also seen in autopsy samples from people who had suffered heart attacks, strokes, and gut ischemia but did not have COVID-19, suggesting that this mechanism could be relevant in many other diseases where ischemia-reperfusion injury occurs.
What This Means for Treatment
This research could open the door to entirely new therapeutic strategies. Targeting the MLKL protein or components of the complement system could potentially prevent or reverse the obstruction of blood vessels in critically ill patients. Alternatively, drugs that help scavenge free heme (the toxic substance released from ruptured red blood cells) might offer another avenue of intervention.
However, the researchers caution that these systems also play important protective roles. Blocking them entirely could increase the risk of uncontrolled bleeding, creating a dangerous trade-off between reducing vessel blockage and maintaining hemostasis.
A New Frontier in Understanding COVID-19 Vascular Damage
In conclusion, this landmark study offers a paradigm-shifting perspective on how COVID-19 and similar ischemic diseases damage the body’s smallest blood vessels. By moving away from the idea that clotting is the central problem and instead pointing to necroptosis-induced hemolysis, it helps to resolve a long-standing mystery in COVID-19 pathology.
The findings show that when blood vessel walls die due to a lack of oxygen, they send out distress signals that cause nearby red blood cells to rupture. The membranes from these lysed cells then stick to the damaged walls, forming a sort of emergency patch to prevent bleeding. But in doing so, they can overaccumulate and physically block blood flow, leading to further organ damage.
This discovery not only explains why anticoagulant treatments often fall short but also hints at potential new drug targets. However, any future treatments must strike a delicate balance between preventing vessel obstruction and preserving the body’s ability to stop internal bleeding. As scientists continue to unravel the long-term consequences of COVID-19, this red cell–based vascular mechanism could prove to be one of the most important pieces of the puzzle yet.
The study findings were published in the peer reviewed journal: Nature
https://www.nature.com/articles/s41586-025-09076-x
For the latest COVID-19 News, keep on logging to
Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-can-trigger-deadly-autoimmune-hemolytic-anemia-which-often-shows-no-early-symptoms
https://www.thailandmedical.news/news/swedish-scientists-discover-strange-blood-cell-gene-activity-in-some-men-with-long-covid
https://www.thailandmedical.news/news/long-covid-and-the-hidden-changes-in-red-blood-cells
https://www.thailandmedical.news/articles/coronavirus
