Glaucoma News: Meta Study Shows That Microbiota Plays Important Role In Glaucoma
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 30, 2023 2 years, 3 months, 2 weeks, 6 days, 14 hours, 40 minutes ago
Glaucoma News: Glaucoma, a prevalent and irreversible vision loss disorder, has been a significant concern in public health due to its gradual progression leading to the loss of retinal ganglion cells (RGCs) and optic nerve axons. The risk factors for glaucoma have long been associated with age and high intraocular pressure (IOP). However, it is crucial to understand that while high IOP is a risk factor, it is neither necessary nor sufficient to cause glaucoma. Additionally, factors like gender, diet, obesity, depression, and anxiety have also been linked to the development of glaucoma. Therefore, it is evident that glaucoma is a multifaceted neurodegenerative disease with various contributing triggers, cell types, and signaling pathways.
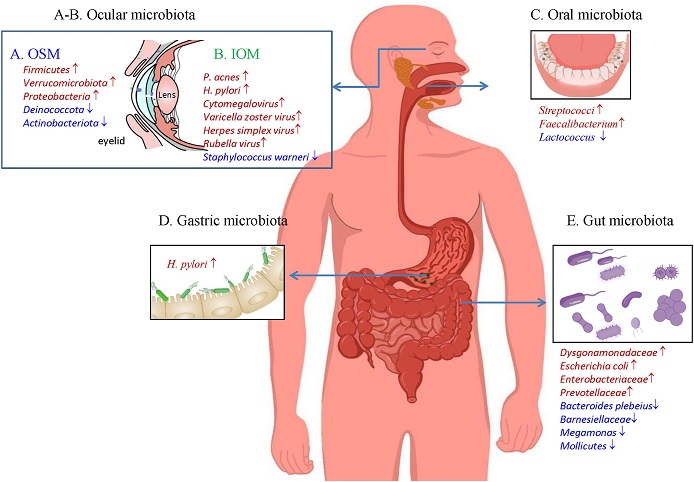 Microbiota changes in five locations [ocular surface (A), intraocular cavity (B), oral cavity(C), stomach (D), and gut (E)] in glaucoma patients. IOM, intraocular microbiota; OSM, ocular surface microbiota.
Microbiota changes in five locations [ocular surface (A), intraocular cavity (B), oral cavity(C), stomach (D), and gut (E)] in glaucoma patients. IOM, intraocular microbiota; OSM, ocular surface microbiota.
One area of increasing interest and research is the role of microbiota in glaucoma. The human body is home to a diverse population of microorganisms, collectively known as the commensal microbiota, residing on various surfaces, including the ocular surface, intraocular cavity, oral cavity, stomach, and gut. Dysbiosis, which refers to an imbalance in the composition, distribution, function, or metabolic activities of these microbiota, has been linked to several human disorders, including autoimmune diseases, inflammatory diseases, neurodegenerative diseases, and ocular diseases like uveitis and diabetic retinopathy. Over the past decade, research has revealed that microbiota play a crucial role in glaucoma and its risk factors. In this comprehensive
Glaucoma News report, we will explore the meta study findings by researchers from West China Hospital-China, Sichuan University-China and the University of Waterloo-Canada related to microbiota and glaucoma, emphasizing its impact on aging, obesity, depression, and the various locations where dysbiosis can occur.
Microbiota and Glaucoma Risk Factors
Aging, Obesity, and Depression
-Aging: Aging is the primary risk factor for glaucoma development. As individuals age, the composition of the gut microbiota undergoes significant changes, resulting in a loss of bacterial diversity. Interestingly, healthy aging is associated with specific changes in the gut microbiota, such as a reduction in core commensal taxa and an increase in pathobionts. This suggests that certain commensal microbiota, including butyrate-producing bacteria, may play a crucial role in aging-related diseases like glaucoma.
-Obesity: Studies have shown a strong link between obesity and primary open-angle glaucoma (POAG). Obese individuals tend to have an altered gut microbiota, characterized by decreased diversity and an increased Firmicutes to Bacteroidetes (F/B) ratio. Gut microbiota dysbiosis in obesity is considered a risk factor for glaucoma.
-Depression: Both anxiety and depression have been associated with an increased risk of glaucoma development. Patients with depression display dysbiosis in t
heir gut microbiota, characterized by the presence of pro-inflammatory species and a reduction in short-chain fatty acid (SCFA)-producing species. SCFAs have been shown to have varying roles in glaucoma pathogenesis.
Changes in Microbiota in Glaucoma
Research in animal models has provided insights into the relationship between microbiota and glaucoma. Mice and rats with glaucoma exhibit altered gut microbiota, including a higher Firmicutes to Bacteroidetes (F/B) ratio and changes in specific bacterial taxa. These changes have been correlated with retinal ganglion cell loss and optic nerve damage.
In human patients, the following were observed:
-Ocular Surface Microbiota (OSM): Studies have revealed differences in the ocular surface microbiota of glaucoma patients, as well as changes associated with the use of anti-glaucoma eye drops. Notably, the composition of the eyelid and ocular surface microbiota varies between glaucoma subtypes, such as open-angle glaucoma (OAG) and uveitic glaucoma. OSM dysbiosis has been linked to the lipopolysaccharide (LPS)-Toll-like receptor 4 (TLR4) pathway, suggesting a mechanism for ocular surface dysbiosis affecting glaucoma.
-Intraocular Microbiota: Traditionally, the intraocular environment of healthy individuals has been considered sterile. However, recent studies have suggested the presence of an intraocular microbiota in humans and certain animals. The presence of Propionibacterium acnes (P. acnes) and other bacteria in the anterior humor of cataract patients has been reported. Studies have also detected differences in the intraocular microbiota between glaucoma patients and non-glaucoma controls, with implications for glaucoma development.
-Oral Microbiota: Dysbiosis in the oral microbiota has been linked to glaucoma. Studies have shown variations in the oral microbiota of glaucoma patients, including increased bacterial loads and changes in specific bacterial species. Additionally, tooth loss and periodontal disorders have been associated with a higher risk of glaucoma.
-Gastric Microbiota: Helicobacter pylori (H. pylori), a gram-negative bacterium that colonizes the stomach, has been linked to glaucoma development. Infection with H. pylori can lead to chronic atrophic gastritis, which results in homocysteine accumulation, inflammation, and direct dissemination to the optic nerve and retina, potentially contributing to glaucomatous damage.
-Gut Microbiota: Studies on the gut microbiota have shown alterations in glaucoma patients, including changes in specific bacterial taxa and microbial metabolites. These alterations have been correlated with visual acuity, visual field mean deviation, and retinal nerve fiber layer thickness, indicating a potential role of the gut microbiota in retinal inflammation and immune damage.
How Microbes Affect Glaucoma Development
-Microbial Metabolites: Microbes in various locations can influence glaucoma development through the production of metabolites. Key microbial metabolites include short-chain fatty acids (SCFAs), bile acids, tryptamine, and histamine. Microbial metabolites can modulate glaucoma development by affecting retinal ganglion cell health, energy metabolism, and inflammatory responses.
-Oral/OSM Dysbiosis and LPS-TLR4 Pathway: Dysbiosis in the oral and ocular surface microbiota can lead to the activation of the lipopolysaccharide (LPS)-Toll-like receptor 4 (TLR4) pathway. This pathway has been implicated in glaucoma pathogenesis, as it can promote inflammatory responses and affect the blood-retinal barrier.
-Antigenic Mimicry of Heat Shock Proteins (HSPs) and Retinal T-Cell Infiltration: Antigenic mimicry, wherein microbial peptides share similarities with host self-antigens, can lead to cross-reactivity and the generation of autoreactive T-cells. This cross-reactivity has been observed in the context of HSPs, highly conserved proteins found in both microbes and humans. Microbial HSPs have been associated with retinal T-cell infiltration and subsequent retinal ganglion cell death, contributing to glaucoma development.
-H. pylori Infection: Helicobacter pylori (H. pylori) infection can impact glaucoma development through the accumulation of homocysteine and inflammation. Elevated homocysteine levels can lead to oxidative stress, damage the optic nerve, and induce retinal ganglion cell apoptosis.
Treatment Targeting the Microbiota of Glaucoma Individuals
Dysbiosis in several locations, including ocular surface, intraocular, oral, gastric, and gut, is a possible mechanism of glaucoma; treatment targeting the microbiota may offer potential help to glaucoma patients. Currently, antibiotics, diet adjustment, and fecal transfer are the three main approaches suggested.
-Antibiotics
Antibiotics, by definition, can inhibit the microbiota. In an OHT mouse model, treatment with a broad-spectrum antibiotic mixture containing ampicillin, metronidazole, neomycin, and vancomycin mitigated the retinal microglia activation. As active gastric H. pylori infection is linked to glaucoma, H. pylori eradication with omeprazole, clarithromycin, amoxicillin, or other antibiotics may benefit glaucoma patients. Oral dysbiosis and oral health are also related to glaucoma. Periodontitis is an inflammatory disorder that progressively destroys the periodontal tissues, resulting in teeth loss. The leading cause of periodontitis is a dental plaque containing pathogenic microorganisms. Antibiotics are suggested for periodontitis in its early stage. OSM of glaucoma eyes had higher anaerobic and gram-negative bacteria of Akkermansia, Faecalibacterium, Lachnospiraceae, and Komagataeibacter. Antibiotic eye drops may be beneficial to these glaucoma patients.
-Diet Modifications
The diet can remodel the gut microbiota structure. Culturally and geographically related dietary diversities may lead to changes in microbiota structure. For example, Firmicutes is enriched in the United States and Russia, Bacteroidetes in France and China, and Prevotella in Germany and India. The MIND diet combines the Mediterranean diet and the DASH diet. The MIND diet significantly reduced the incidence of OAG and was IOP-independent. Thus, the MIND diet may help prevent glaucomatous neurodegeneration. However, a USA study found no overall benefit of low-fat diet modification with more fruits, vegetables, and grains in reducing POAG among women. Surprisingly, reducing fat intake in some participants was harmful and increased POAG incidence, suggesting that maintaining a healthy diet, including adequate fat intake, is crucial in preventing POAG. More studies are needed to study the gut microbiota changes under a MIND or low-fat diet.
-Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) transports fecal material from a healthy donor into the gastrointestinal tract of patients to replace their dysbiotic microbiota at the compositional and functional levels. FMT has been proven to be efficacious in treating ulcerative colitis by modulating the fecal and mucosal gut microbiota. Fecal contents from glaucoma patients and healthy controls were transferred to C57BL/6 J mice, pre-treated with an antibiotic cocktail. FMT with glaucoma fecal materials activated retinal microglia and promoted the retinal inflammatory reaction, which led to retinal cell loss in the OHT mouse. However, whether FMT from healthy donors can reduce glaucomatous RGC loss and optic nerve damage has not been tested.
Conclusion
The evidence gathered from various studies suggests that microbiota play a substantial role in the pathogenesis of glaucoma and its associated risk factors, such as aging, obesity, and depression. Dysbiosis in different microbiota locations, including the gut, oral cavity, ocular surface, and intraocular environment, can lead to glaucomatous damage through various mechanisms, including microbial metabolites, inflammatory pathways, antigenic mimicry, and direct infection.
Understanding the connection between microbiota and glaucoma holds significant promise for developing novel therapeutic strategies. Targeted interventions, such as prebiotics, probiotics, and specific dietary modifications, may help restore a balanced microbiota and mitigate glaucoma risk. Moreover, developing personalized treatment approaches based on an individual's microbiota composition could revolutionize glaucoma management. However, further research is needed to elucidate the precise mechanisms and potential interventions, making microbiota an exciting and evolving frontier in glaucoma research and treatment.
This comprehensive meta-study of the role of microbiota in glaucoma reinforces the idea that the complex interplay between the human body and its microbial inhabitants is crucial to our understanding of this neurodegenerative disease. It emphasizes the need for a holistic approach to healthcare, where factors such as diet, mental health, and microbiota composition are considered in the diagnosis and management of glaucoma. The future of glaucoma research and therapy might well be in the hands of the microbiota.
The study findings were published in the peer reviewed journal: Molecular Aspects of Medicine.
https://www.sciencedirect.com/science/article/pii/S0098299723000614
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
