COVID-19 News: Study Finds That Omicron Variants Causes Outcomes In Blood Platelets Similar To Delta If Not Worse But Via Different Mechanisms!
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 27, 2023 2 years, 4 months, 3 weeks, 5 days, 18 hours, 14 minutes ago
COVID-19 News: The COVID-19 pandemic has witnessed the emergence of various SARS-CoV-2 variants, each with its own set of mutations and characteristics. Among these, the Omicron variant, identified in November 2021, rapidly gained notoriety for its increased transmissibility and global dominance. While Omicron has been associated with milder clinical symptoms compared to earlier variants, its impact on blood platelets has remained an enigma.
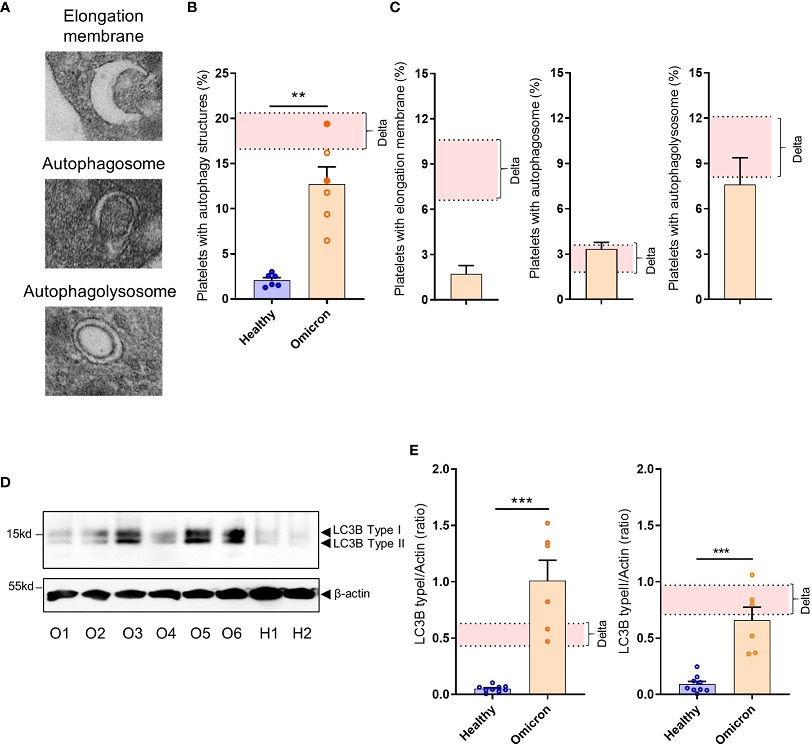 Platelets from severe COVID-19 patients infected with the Omicron variant have autophagic structures. Representative TEM sections of platelets from severe COVID-19 patients infected with the Omicron variant highlighting structures typical of elongation membrane (EM), autophagosome (AP), and autophagolysosome-like (AL) are shown (A). The percentage of platelets containing autophagy structures is presented. Each circle represents an individual (full circle: seropositive, empty circle: seronegative) and results are mean ± SEM (B) along with the frequency of occurrence of the different types of structures (C) in platelets from 6 healthy donors and 6 severe COVID-19 patients with the Omicron variant. Representative Western blotting analysis of LC3B-I and LC3B-II levels in platelets from 6 severe COVID-19 patients with the Omicron variant (O1 to O6) and 9 healthy donors (H1, H2) are shown (D). The exposure time was adjusted in order to efficiently distinguish the LC3B type I and type II. The quantification of the LC3B-I/actin ratio and LC3B-II/actin ratio for healthy donors (n=9), and patients with the Omicron variant (n=6) is shown (E). Significance denoted as **p <.01 and ***p <.001 represents the comparison of healthy donors vs. Omicron patients, based on the nonparametric Mann-Whitney test. The pink areas in the graphs represents the mean +/- SEM we previously found in platelets from severe patients with the Delta variant.
Platelets from severe COVID-19 patients infected with the Omicron variant have autophagic structures. Representative TEM sections of platelets from severe COVID-19 patients infected with the Omicron variant highlighting structures typical of elongation membrane (EM), autophagosome (AP), and autophagolysosome-like (AL) are shown (A). The percentage of platelets containing autophagy structures is presented. Each circle represents an individual (full circle: seropositive, empty circle: seronegative) and results are mean ± SEM (B) along with the frequency of occurrence of the different types of structures (C) in platelets from 6 healthy donors and 6 severe COVID-19 patients with the Omicron variant. Representative Western blotting analysis of LC3B-I and LC3B-II levels in platelets from 6 severe COVID-19 patients with the Omicron variant (O1 to O6) and 9 healthy donors (H1, H2) are shown (D). The exposure time was adjusted in order to efficiently distinguish the LC3B type I and type II. The quantification of the LC3B-I/actin ratio and LC3B-II/actin ratio for healthy donors (n=9), and patients with the Omicron variant (n=6) is shown (E). Significance denoted as **p <.01 and ***p <.001 represents the comparison of healthy donors vs. Omicron patients, based on the nonparametric Mann-Whitney test. The pink areas in the graphs represents the mean +/- SEM we previously found in platelets from severe patients with the Delta variant.
Previous studies and
COVID-19 News coverages in the West claimed that infections by the Omicron variant were milder and there were lesser deaths, but this new study and also many other recent studies are dispelling these fallacies and are showing that the Omicron variants are better for longer viral persistence in the human hosts, causing extensive damage and deaths much later unlike the Delta variant that causes immediate disease severity and deaths!
A new groundbreaking study conducted by French researchers from Inserm UMR1297, Université Toulouse-France, and Centre Hospitalier Universitaire de Toulouse-France, sheds light on how the Omicron variant affects blood platelets and how this compares to the Delta variant.
Blood platelets, tiny cellular elements, play a pivotal role in maintaining normal hemostasis, responding to vascular injuries, and contributing to thrombosis. However, their involvement in COVID-19 pathology has been increasingly recognized, especially in severe cases. Platelet activation during severe COVID-19 has been linked to thromboembolic events and exacerbated inflammatory responses, including acute respiratory distress syndrome (ARDS).
Platelets in the Context of COVID-19
Platelet activation during COVID-19 is a multifaceted phe
nomenon that can contribute to the severity of the disease. Several studies have reported platelet hyperactivation and their interaction with other immune cells, particularly monocytes. Post-mortem analyses of pulmonary endothelium have revealed platelet-rich thrombi, further emphasizing the role of platelets in COVID-19 pathogenesis.
Interestingly, despite the relatively low presence of angiotensin-converting enzyme 2 (ACE2), the primary receptor for SARS-CoV-2, in platelets, these tiny cellular components have been shown to contain viral particles and can internalize the virus. This internalization occurs through various pathways, adding to the complexity of platelet-virus interactions. Moreover, SARS-CoV-2 infection has been found to modify the transcriptome of platelets, triggering an antiviral response phenotype.
The Rise of the Omicron Variant
The Omicron variant distinguishes itself from its predecessors with a notable accumulation of mutations, especially in the spike protein, which is a key element for viral entry into host cells. These mutations have enhanced Omicron's transmissibility potential, and initial reports suggested that it induces milder disease symptoms. Omicron's entry into host cells also appears to be independent of the transmembrane serine protease 2 (TMPRSS2), which hints at differences in viral behavior and internalization mechanisms.
Despite the growing knowledge about Omicron's characteristics, its effects on blood platelets have remained a subject of inquiry. This study sought to bridge that gap and provide insights into platelet behavior in severe COVID-19 patients infected with the Omicron variant, comparing it to the Delta variant.
Study Methodology and Patient Characteristics
The study involved the analysis of clinical and biological data from severe COVID-19 patients infected with either the Omicron or Delta variants, with a total of 20 patients included in the study. The patients' severity was assessed using the Sequential Organ Failure Assessment (SOFA) score, and the study included 10 healthy donors as controls for comparison.
Upon analysis, it was observed that the Omicron-infected patients generally exhibited a less severe clinical course compared to those infected with the Delta variant, as evidenced by the SOFA scores. However, this distinction in severity did not translate to significantly lower mortality rates. Both patient groups showed elevated levels of pro-inflammatory cytokines, emphasizing the persistent inflammatory response associated with SARS-CoV-2 infection.
Platelet Characteristics in Omicron-Infected Patients
The study delved into several aspects of platelet behavior in patients infected with the Omicron variant. Surface expression of key platelet glycoproteins revealed a shedding process, with decreased expression of GPIaIIa, GPIIbIIIa (αIIbβ3), and GPVI. This phenomenon indicated platelet activation and was further substantiated by electron microscopy analysis, which displayed signs of prior platelet activation.
Soluble platelet activation markers, such as sGPVI, sCD62P, and sCD40L, were significantly increased in the plasma of Omicron-infected patients compared to controls. While resting platelets showed no significant differences in activation markers compared to controls, platelet stimulation with various agonists led to increased membrane expression of CD62P and CD63, along with αIIbβ3 integrin activation. However, this response was less pronounced in platelets from Omicron-infected patients, indicating partial platelet hyporesponsiveness, which had previously been observed in severe Delta infection.
The Omicron Variant and Platelet Viral Infection Response
Intriguingly, the study revealed that the Omicron variant induced an antiviral response phenotype in platelets. The expression of IFITM3, an interferon-induced transmembrane protein known to inhibit viral replication, was elevated in platelets from Omicron-infected patients, although to a lesser extent compared to Delta-infected patients. This finding suggests that platelets play a role in the antiviral response triggered by Omicron.
Additionally, the study explored Toll-like receptor (TLR) activation in platelets. While platelets from Delta-infected patients exhibited upregulated and phosphorylated IRAK4, reflecting TLR activation, platelets from Omicron-infected patients showed weaker upregulation and phosphorylation of IRAK4. This distinction suggests that the two variants trigger different levels of TLR activation in platelets.
Viral Presence in Platelets: Omicron vs. Delta
A key finding of the study was the presence of viral-like particles within platelets from severe Omicron-infected patients. Approximately 27.7% of platelets in these patients contained viral-like particles, a proportion slightly higher than that observed in Delta infection. This observation underscores the ability of the Omicron variant to infect and reside within platelets.
Distinct Mechanisms of Entry and Processing
Super-resolution confocal microscopy revealed that the distribution of spike protein in platelets differed between the two variants. In Omicron-infected patients, spike protein was observed near the plasma membrane, suggesting a unique mechanism of entry and/or processing in platelets compared to Delta infection. The colocalization of spike protein with Rab7, an important regulator of late endosome function, was significantly increased in platelets from Omicron-infected patients, further indicating differences in intraplatelet processing of viral cargo.
Effect of Spike Proteins on Platelet Signaling
In vitro experiments with recombinant spike proteins showed that both Omicron and Delta spike proteins could induce platelet activation. However, they exhibited distinct kinetics in activating signaling pathways, with the Omicron spike protein eliciting a much faster response compared to Delta. This observation suggests that the mutations present in the Omicron spike protein modify its interaction with platelets and the resulting signaling cascades.
Implications and Future Directions
This groundbreaking study provides valuable insights into the interaction between the Omicron variant of SARS-CoV-2 and blood platelets. While Omicron is associated with milder clinical symptoms, it induces platelet activation, partial desensitization, and the presence of viral particles within platelets, much like the Delta variant. However, the study highlights that platelets have evolved different mechanisms to respond to the two variants, indicating the dynamic nature of the virus-platelet interaction.
Understanding how platelets respond to different SARS-CoV-2 variants is essential not only for deciphering the thrombotic potential but also for comprehending their antiviral and immune functions. These findings have implications for potential therapeutic strategies targeting the virus-platelet interaction. As SARS-CoV-2 continues to evolve through mutations, ongoing research is crucial to adapt our understanding of its interaction with the host immune system, including blood platelets. This knowledge is instrumental in devising effective treatments and interventions to combat the ever-changing landscape of the COVID-19 pandemic.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1231576/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
