BREAKING! Rush University Medical Centre’s Silico And Vivo Study Shows That Eugenol From Holy Basil (Tulsi) And Cloves Is Effective Against COVID-19
Source: Thailand Medical Nov 08, 2021 4 years, 3 months, 6 days, 2 hours, 15 minutes ago
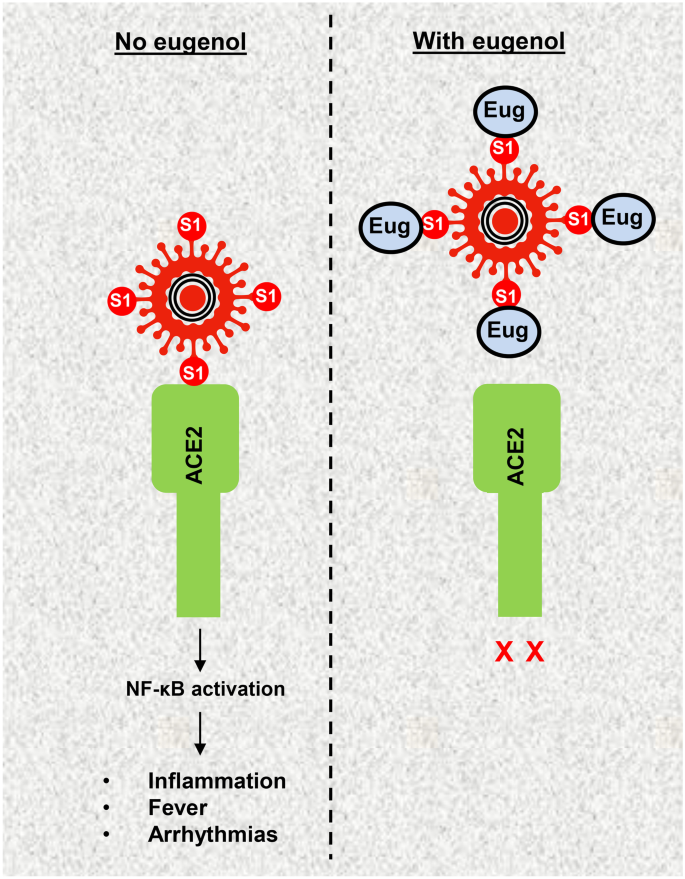
A new silico and vivo study led by scientist from Rush University Medical Centre-Chicago, USA along with the support from Northwestern University, Chicago has shown that the phytochemical Eugenol extracted from Holy Basil (Also known as Tulsi in India) and Cloves is effective against the COVID-19 disease. The study found that Eugenol inhibited the interaction between spike S1 and ACE2.

The study findings could pave the way for Eugenol to be developed into a therapeutic product to treat COVID-19.
It is already known that the spike S1 of the SARS-CoV-2 virus binds to angiotensin-converting enzyme 2 (ACE2) on host cells in order to enter the cell and initiate COVID-19.
Since ACE2 is a favorable enzyme, the study team was interested in finding a molecule capable of binding spike S1, but not ACE2, and inhibiting the interaction between spike S1 and ACE2.
Holy basil (Tulsi) has a long history as a medicine for different human disorders. Hence the study team screened different components of Tulsi leaf and found that eugenol, but not other major components (e.g. ursolic acid, oleanolic acid and β-caryophylline), inhibited the interaction between spike S1 and ACE2 in an AlphaScreen-based assay.
Utilizing silico analysis and thermal shift assay, the study team also observed that eugenol associated with spike S1, but not ACE2. Accordingly, eugenol strongly suppressed the entry of pseudotyped SARS-CoV-2, but not vesicular stomatitis virus (VSV), into human ACE2-expressing HEK293 cells. Eugenol also reduced SARS-CoV-2 spike S1-induced activation of NF-κB and the expression of IL-6, IL-1β and TNFα in human A549 lung cells.
Importantly, oral treatment with eugenol reduced lung inflammation, decreased fever, improved heart function, and enhanced locomotor activities in SARS-CoV-2 spike S1-intoxicated mice. Therefore, selective targeting of SARS-CoV-2 spike S1, but not ACE2, by eugenol may be beneficial for COVID-19 treatment.
The study findings were published in the peer reviewed Journal of Neuroimmune Pharmacology.
https://link.springer.com/article/10.1007/s11481-021-10028-1
The herb Holy basil or Tulsi is mainly cultivated for religious and traditional medicine purposes in India and also elsewhere in Asia.
The study findings showed that the phytochemical Eugenol from Holy Basil and Cloves successfully controls lung inflammation, decreases fever, and recovers heart function in a mouse model of COVID-19.
Dr Kalipada Pahan, PhD, the Floyd A. Davis Professor of Neurology at Rush and a Research Career Scientist at the Jesse Brown VA Medical Center told
Thailand Medical News, “Understanding the mechanism is important to developing effective therapy for COVID-19.”
As studies have shown that the spike S1 of SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) in order to enter into the human host cells, the study team screened different components of the Tulsi leaf based on inhibition of such binding.
e="height:475px; width:370px" />
Graphical Abstract
Dr Pahan added, “Interestingly, eugenol, but not other major constituents (e.g. ursolic acid, oleanolic acid and β-caryophylline), of tulsi leaf inhibits the interaction between ACE2 and viral spike S1. Eugenol also effectively prevents viral entry into human cells.”
Dr Pahan further added, “A good COVID-19 therapeutic agent or drug should not bind and inhibit ACE2 as it is a beneficial molecule. It is nice to see that eugenol binds to spike S1, not ACE2.”
The study team demonstrated in vivo studies involving murine models infected with COVID-19 that after oral treatment, eugenol reduces fever, decreases lung inflammation, normalizes heart function, and enhances locomotor activities.
It should be noted that in addition to Holy Basil or Tulsi,
Eugenol is naturally available in Clove, Ginger, Celery, etc but in smaller quantities.
Dr Pahan added, “Therefore, oral eugenol could be a safer approach than available options to prevent SARS-CoV-2 infection and protect COVID-19 patients from various complications.”
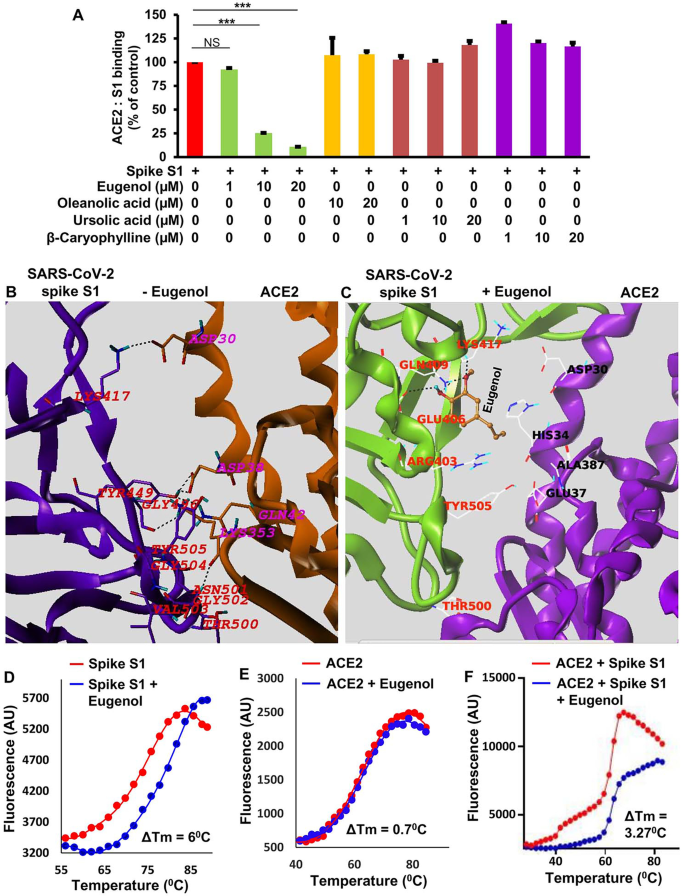 Eugenol is capable of inhibiting the interaction between ACE2 and SARS-CoV-2 spike S1. A) Chemiluminescence assay indicated inhibition of ACE2 to SARS-CoV-2 spike S1 interaction by different doses of eugenol, but not ursolic acid, oleanolic acid and β-caryophylline (other major components of Tulsi leaf). ***p < 0.001; NS, not significant. B) A rigid-body in silico docked pose of human ACE2 (dark yellow epoxy color) and SARS-CoV-2 spike S1 (dark magenta) in the absence of eugenol. C) A rigid-body in silico docked pose of human ACE2 (magenta) and SARS-CoV-2 spike S1 (green) in the presence of eugenol (dark yellow structure). D) Thermal shift assay of spike S1 was conducted with 10 µM eugenol. The melting of spike S1 was monitored using a SYBR Green real-time melting strategy. E) Thermal shift assay of human ACE2 was conducted with 10 µM eugenol. F) Thermal shift assay of the combination of spike S1 and human ACE2 was conducted with 10 µM eugenol. Results were analyzed and confirmed after three independent experiments
Eugenol is capable of inhibiting the interaction between ACE2 and SARS-CoV-2 spike S1. A) Chemiluminescence assay indicated inhibition of ACE2 to SARS-CoV-2 spike S1 interaction by different doses of eugenol, but not ursolic acid, oleanolic acid and β-caryophylline (other major components of Tulsi leaf). ***p < 0.001; NS, not significant. B) A rigid-body in silico docked pose of human ACE2 (dark yellow epoxy color) and SARS-CoV-2 spike S1 (dark magenta) in the absence of eugenol. C) A rigid-body in silico docked pose of human ACE2 (magenta) and SARS-CoV-2 spike S1 (green) in the presence of eugenol (dark yellow structure). D) Thermal shift assay of spike S1 was conducted with 10 µM eugenol. The melting of spike S1 was monitored using a SYBR Green real-time melting strategy. E) Thermal shift assay of human ACE2 was conducted with 10 µM eugenol. F) Thermal shift assay of the combination of spike S1 and human ACE2 was conducted with 10 µM eugenol. Results were analyzed and confirmed after three independent experiments
Currently, Eugenol is under clinical development and Dr Pahan hopes his team’s new treatment will control infection in individuals who haven’t been vaccinated and that it will be useful in the event a new strain of the virus emerges that eludes vaccines’ protection.
Thailand Medical News is collaborating with Dr Pahan’s team on a variety of herbal and phytochemical based therapeutics.
As the COVID-19 pandemic continues to wreak havoc globally despite a massive vaccination campaign, emerging SARS-CoV-2 variants and sub-variants that are getting to be even more immune evasive, more transmissible and even more potent and virulent are emerging and there is a dire need to shift the focus back on antivirals and therapeutics that can control the virus and infections. The medical and scientific community is finally coming to terms that herbs and phytochemicals are the best option considering that many are safe and have been used for eons and that in the case of the SARS-CoV-2 which is ever mutating and evolving, a combination of such herbs and phytochemicals would be a more viable approach.
Dr Pahan’s Lab is a research laboratory at the Rush University Medical Center. The other study team members include Dr Ramesh K. Paidi, Dr Malabendu Jana, Dr Sumita Raha, Mary McKay, and Monica Sheinin from Rush University Medical Center and Dr Rama K. Mishra from Northwestern University.
Thailand Medical News would however like to warn readers to be wary of herbal or phytochemical products produced in India, even by so called GMP certified factories that are being touted to treat COVID-19 or Long COVID. Most are basically fraud products with no efficacy and some have even resorted to buying unethical researchers and doctors in India to conduct sub-standard clinical trials and manipulate clinical trial results in order to peddle their sub-standard products that they claim can treat COVID-19 or Long COVID.
Furthermore, many therapeutic products from India are either contaminated or adulterated. Last month in October alone, there were two main issues involving products from India and this month, there are about 17 issues that will be published in the mainstream media in coming days.
https://ktla.com/news/nationworld/aromatherapy-spray-imported-from-india-and-sold-at-walmart-linked-to-u-s-deaths/
https://www.npr.org/2021/10/20/1047658921/blood-pressure-medication-recall-cancer-irbesartan-hydrochlorothiazide
For the latest on
COVID-19 Herbs, keep on logging to
Thailand Medical News.

 e="height:475px; width:370px" />
e="height:475px; width:370px" />