BREAKING! Study Alarmingly Shows Omicron Could Be Possibly Evolving To Shift Its Focus On Human Receptors From ACE2 To DDP-IV, Similar To MERs!.
Source: Omicron Research - DDP4 Dec 21, 2021 4 years, 1 month, 3 weeks, 3 days, 3 hours, 58 minutes ago
Omicron Research: A new study by researchers from the University Medicine Rostock-Germany has alarmingly found that the Omicron variant could be evolving to switch its focus from binding with the human host receptor ACE2 to other receptors found in the human host such as the DDP-IV receptor, which is the entry receptor of the MERs-CoV virus!
Dear readers, we are appealing for your kind help and support this Christmas and the coming new year. We are desperately needing funds to sustain this website and all our research and community initiatives and need your help. Please donate. Thank You. https://www.thailandmedical.news/p/sponsorship
The study found that in general, the Omicron variant had a weaker bindings affinity for the ACE2 receptor unlike the original Wuhan wildtype strain.
The study focused on free energy calculations of SARSCoV2 spike protein receptor binding motives (RBMs) from wild type and VOCs or variants of concern with particular emphasis on currently emerging SARS-CoV-2 omicron variants of concern (VOC).
The computational free energy analysis underlines the occurrence of positive selection processes that specify omicron host adaption and bring changes on the molecular level into context with clinically relevant observations.
The study team’s free energy calculations studies regarding the interaction of omicron´s RBM with human ACE2 shows weaker binding to ACE2 than alpha´s, delta´s, or wild type´s RBM. Thus, less virus is predicted to be generated in time per infected cell. The mutant analyses predict with focus on omicron variants a reduced spike-protein binding to ACE2receptor protein possibly enhancing viral fitness / transmissibility and resulting in a delayed induction of danger signals as tradeoff.
Worryingly as more virus is produced but less per cell, this could lead to delayed COVID-19 immunogenicity and pathogenicity. Regarding the latter, more virus is assumed to be required to initiate inflammatory immune responses.
The study also found that by contrast, the free energy difference calculations or ΔΔG value differences of RBD- DPP-IV binding of all VOCs showed that all their respective complexes were bound with weaker forces than that of wild type SARS-CoV-2.
Interestingly, the omicron RBM binding with human DPP-IV requests a smaller increase in free energy (ΔΔG: +3.66 kJ/mol) than that of omicron RBM binding with human ACE2 (ΔΔG: +5.93 kJ/mol) when taking the N417k mutation exchange into account.
This could imply that such a free energy difference was large enough to cause omicron to switch host receptors, hence, to possibly alter tropism and to eventually afford different disease symptoms.
The study findings were published on a preprint server and are currently being pee
r reviewed.
https://www.biorxiv.org/content/10.1101/2021.12.14.472585v1
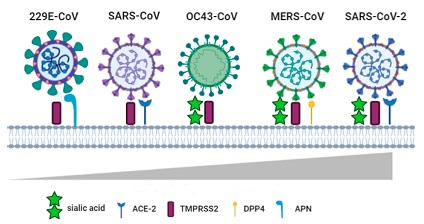
The DDP-IV or Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 (cluster of differentiation 26) is a protein that, in humans, is encoded by the DPP4 gene. The protein encoded by the DPP4 gene is an enzyme expressed on the surface of most cell types and is associated with immune regulation, signal transduction, and apoptosis. It is a type II transmembrane glycoprotein, but a soluble form, which lacks the intracellular and transmembrane part, is present in blood plasma and various body fluids.
DPP-4 is known to cleave a broad range of substrates including growth factors, chemokines, neuropeptides, and vasoactive peptides. The cleaved substrates lose their biological activity in the majority of cases, but in the case of the chemokine RANTES and neuropeptide Y, DPP-4 mediated cleavage leads to a shift in the receptor subtype binding.
DPP4 plays a major role in glucose metabolism. It is responsible for the degradation of incretins such as GLP-1. Furthermore, it appears to work as a suppressor in the development of some tumors.
Most importantly the DDP-IV receptors are the entry receptors used by the MERs or Middle East Respiratory Syndrome Coronavirus to gain entry into the human host!
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3731569/
Study Details
The B.1.1.529 or Omicron variant has recently risen to prominence, sparking several countries’ return to more severe social distancing measures and restrictions designed to reduce transmission.
The new Omicron strain carries several mutations in the spike protein. The spike protein is key to the pathogenicity of the disease; the S1 subunit carries a receptor-binding domain (RBD) that binds to the angiotensin-converting enzyme in order to allow cell entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), while the S2 subunit is responsible for membrane fusion.
Dear readers, we are appealing for your kind help and support this Christmas and the coming new year. We are desperately needing funds to sustain this website and all our research and community initiatives and need your help. Please donate. Thank You. https://www.thailandmedical.news/p/sponsorship
The
Omicron Research team from the University of Rostock have been examining the effects of some of these mutations.
The receptor-binding domain or RBD of the wild-type SARS-CoV-2 spike protein shows the amino acid sequence aa437 to aa508 stretch enough to encompass the receptor-binding motif (RBM).
It has been reported that in VOCs, amino acid residue exchanges can be seen at distinct positions with the RBM. For example, the omicron variant shows ten exchanged amino acids, five of which can also be found in severe acute respiratory syndrome (SARS-CoV-1/SARS). Through these changes, the RBM of the Omicron variant can be distinguished from the wild-type. 3D structures from both SARS-CoV-1 and SARS-CoV-2 are revealed using X-ray data, while the RBM structure is modeled using alphafold. The five RBM amino acid exchanges that are not also found in SARS-CoV-1 or the wild-type are unique to Omicron.
Interestingly one of the mutations Omicron carries is Y501, which strengthens the binding to ACE2, and can be found in the Alpha, Beta, and Gamma variants.
Residue K4768 has been designated as the decisive amino acid exchange for the Delta variant.
Importantly this exchange remains in Omicron, and alongside residues K440, S446 and N447, these changes lend Omicron characteristics not seen in other variants – for example, K478 matching with K465, which is more characteristic of SARS-CoV-1 than SARS-CoV-2.
In the Alpha variant, the residue E484 is known to weaken receptor binding. Omicron avoids this by expressing A484, matching the equivalent SARS-CoV-2 residue A471 – located next to the L472 residue that comes into direct contact with ACE2.
Significantly, another residue, K493, in the RBM of Omicron is positioned where N479 is found in SARS-CoV-1. N479 is another residue that contacts ACE2 and is also considered essential for binding and infectivity.
Importantly an N479K exchange results in steric hindrance and weakening of ACE2 binding. S496 and R498 are rare mutations in the RBM, likely as they are assumed to have adverse effects on binding due to the introduction of charge repulsion. Y501 is thought to strengthen binding and increase replication rates. H505 is located where Y491 is on SARS-CoV-1 – replacing Y505 seen in the wild-type. Y505 is directly involved in hACE2 binding, and the residue exchange is assumed to weaken binding.
The study team performed free energy difference calculations to confirm their hypothesis: that the Omicron RBM binding to ACE2 was weaker than the wild-type.
The study team examined the energy difference for the RBMs if either the wild-type, Alpha, Delta or Omicron variants were bound to ACE2, and compared them to respective RBM DPP-IV interactions as a negative control. According to ΔΔG calculations on amino acid exchanges and their contributions to receptor binding, the Alpha RBM is the only variant that should show stronger binding to ACE2 than the wild-type. The Delta RBM shows roughly equivalent binding strength compared to the wild-type, but the Omicron RBM-ACE2 complex is less energetically favored.
The study findings showed that Omicron binds to the ACE2 receptor in a significantly weaker manner than other variants and the wild-type.
The study team suggest that the increased transmission of Omicron results from mutations outside of the RBM, and imply that the virus could be possibly beginning to adapt more to the host, with less severe disease outcomes and higher transmission.
Alarmingly however, there are some issues ie the negative control used can be bound by SARS-CoV-2, making it inappropriate for use in this circumstance. While the binding of the Omicron variant appears to be lower, this has not been confirmed by more physical experimentation, such as binding assays, and the conclusions may somewhat overreach the evidence gathered. The best example of this would be the assumptions the authors make of the severity of disease outcome of which there is currently very little solid data compared to RBM-ACE2 binding. There is currently little indication that a change of this size in RBM binding and disease severity are significantly linked.
Furthermore, the Omicron could be shifting to bind to other receptors including the DDP-IV receptors, this could result in more long-term health issues and medical conditions.
https://www.thailandmedical.news/news/good-news-preliminary-unpublished-data-shows-that-omicron-might-not-affect-lungs-much,-rather-your-brains,-heart-and-other-organs-are-targets
Dear readers, we are appealing for your kind help and support this Christmas and the coming new year. We are desperately needing funds to sustain this website and all our research and community initiatives and need your help. Please donate. Thank You. https://www.thailandmedical.news/p/sponsorship
For the latest
Omicron Research, keep on logging to Thailand Medical News.
