Medical News: Scientists Discover Protein Called TAF15 That Causes Early Onset Dementia!
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 12, 2023 2 years, 2 months, 2 days, 1 hour, 4 minutes ago
Medical News: Frontotemporal dementia (FTD) has long perplexed researchers due to its heterogeneity and, in about 10% of cases, the absence of a known culprit protein. A groundbreaking study covered in this
Medical News report, led by scientists at the Medical Research Council (MRC) Laboratory of Molecular Biology in Cambridge, UK, has illuminated this elusive aspect of FTD, identifying a novel protein, TAF15, and uncovering its involvement in the neurodegenerative process. This discovery not only transforms our understanding of FTD's molecular basis but also holds promising implications for diagnostic advancements and targeted treatments.
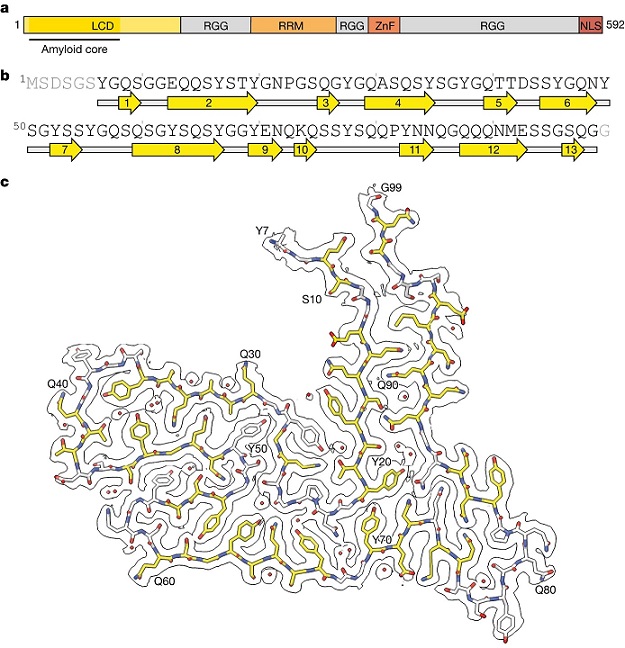 Cryo-EM structure of TAF15 amyloid filaments from FTLD–FET.
Cryo-EM structure of TAF15 amyloid filaments from FTLD–FET.
a, Domain organization of TAF15. The region comprising the ordered core of TAF15 amyloid filaments is indicated. RRM, RNA recognition motif; ZnF, zinc finger domain. b, Sequence alignment of secondary structure elements of the TAF15 amyloid filament fold. Arrows indicate β-strands. c, Cryo-EM reconstruction and atomic model of the TAF15 amyloid filament structure, shown for a single TAF15 molecule perpendicular to the helical axis. The carbon atoms of residues forming β-strands are shown in yellow and ordered solvent as red spheres.
Frontotemporal Dementia: A Complex Neurodegenerative Disorder
FTD is a form of dementia characterized by the degeneration of the frontal and temporal lobes of the brain. These areas govern emotional control, personality, behavior, speech, and word comprehension. Unlike Alzheimer's disease, FTD tends to strike at a relatively younger age, affecting individuals between 45 and 65, although it can manifest in both younger and older age groups.
Historical Challenges and the Quest for the Rogue Protein
In most neurodegenerative diseases, including FTD, proteins aggregate into filaments known as amyloids. Identifying these proteins has been crucial for developing diagnostic tests and treatment strategies. However, in approximately 10% of FTD cases, the identity of the rogue protein had remained elusive. The recent study led by Dr Benjamin Ryskeldi-Falcon and his team at the MRC Laboratory of Molecular Biology has shed light on this mystery.
Cutting-Edge Technology: Cryo-Electron Microscopy
To unravel the molecular complexities of FTD, the researchers employed cutting-edge cryo-electron microscopy (cryo-EM). This technology allowed them to examine brain tissues from four individuals diagnosed with this specific form of dementia at an atomic resolution. The brains were generously donated, and the study benefited from the expertise of Tammaryn Lashley at the University College London Queen Square Institute of Neurology and Bernardino Ghetti at the Indiana University School of Medicine.
Unexpected Revelation: TAF15 Takes Center Stage
Contrary to expectations, the aggregated structures identified through cryo-EM were not composed of the previously suspected FUS protein. Instead, the researchers discovered that TAF15, a protein not previously k
nown for forming amyloid filaments in neurodegenerative diseases, was the key player in this subset of FTD cases. Dr Stephan Tetter, a co-author of the study, emphasized the unexpected nature of this finding, highlighting how cryo-EM is transforming the understanding of molecular pathology in dementia.
The Structural Insight: TAF15 Amyloid Filaments
The study delved into the atomic structure of TAF15 amyloid filaments, revealing that they comprise residues 7–99 in the low-complexity domain (LCD) of TAF15, forming a left-handed helical twist. This characteristic fold, enriched in glycine, tyrosine, glutamine, and serine residues, exhibited parallel, in-register β-sheets—a hallmark of amyloid filaments. The detailed structural understanding obtained through cryo-EM provides a foundation for future model systems and the development of diagnostic and therapeutic tools targeting TAF15 proteinopathy.
Implications for Frontotemporal Dementia and Motor Neuron Disease
Beyond FTD, the study explored the connection between TAF15 and motor neuron disease, a condition involving the progressive loss of muscle control. Two individuals whose brains were analyzed in the study exhibited signs of both FTD and motor neuron disease. The shared aggregated structure of TAF15 in brain regions associated with motor neuron disease raises intriguing possibilities about TAF15's potential contribution to both conditions.
Decades of Research Excellence: The MRC Laboratory of Molecular Biology
The MRC Laboratory of Molecular Biology has been at the forefront of groundbreaking research, contributing significantly to the understanding of neurodegenerative diseases. Cryoelectron microscopy, a revolutionary technique that earned Dr Richard Henderson a Nobel Prize in 2017, has been pivotal in elucidating the structures of key proteins associated with dementia. Dr Charlotte Durkin, Head of the Medical Research Council's Molecular and Cellular Medicine Board, highlighted the laboratory's success in revealing structures such as tau, a key protein in Alzheimer's disease, and now, TAF15 in FTD.
Broader Implications: From FTLD–FET to Potential FTLD–TAF
The study's findings have broader implications for the nomenclature of the disease. Dr Ryskeldi-Falcon advocated abandoning the frequently used term FTLD–FUS in favor of FTLD–FET, or even considering the use of FTLD–TAF. This suggested shift in terminology reflects the evolving understanding of the molecular underpinnings of neurodegenerative diseases and emphasizes the need for precision in disease classification.
Genetic Analysis and Future Research Avenues
The identification of TAF15 in FTD raises questions about the genetic landscape of this subset of cases. While rare mutations in genes encoding proteins like TDP-43 and tau are associated with inherited forms of FTD, the role of TAF15 mutations remains uncertain. The study's authors advocate for genetic analysis of patient cohorts to determine the potential contribution of rare TAF15 mutations to FTD and motor neuron disease.
Distinct Filament Fold: TAF15's Unique Signature
Unlike other neurodegenerative disorders characterized by distinct filament folds for proteins like TDP-43, tau, and α-synuclein, the study revealed a single TAF15 filament fold in FTLD–FET. This unique signature suggests that TAF15 may play a central role in the pathology of this specific form of FTD, marking a departure from the familiar patterns observed in other proteinopathies.
Therapeutic Potential and Hope for Affected Individuals
The identification of TAF15 opens new avenues for therapeutic interventions, building on the progress made in developing treatments for other neurodegenerative diseases. Dr Ryskeldi-Falcon expressed optimism about the potential development of tools to screen hundreds of patient samples, assessing the prevalence of abnormal TAF15 aggregates. This could lead to the development of targeted therapies, offering hope to individuals affected by FTD and related motor neuron diseases.
Conclusion
The groundbreaking discovery of TAF15's involvement in frontotemporal dementia represents a significant leap forward in our understanding of this complex neurodegenerative disorder. Cryo-electron microscopy has played a pivotal role in unraveling the atomic structure of TAF15 amyloid filaments, providing a foundation for future research and therapeutic development. As the scientific community delves deeper into the genetic aspects of TAF15 and its potential role in inherited forms of FTD and motor neuron disease, the prospects of diagnostic advancements and targeted treatments are becoming increasingly promising. The evolving nomenclature, from FTLD–FUS to FTLD–FET or even FTLD–TAF, reflects the dynamic nature of research in neurodegenerative diseases, offering renewed hope for individuals affected by these devastating conditions.
The study findings were published in the peer reviewed journal: Nature.
https://www.nature.com/articles/s41586-023-06801-2
For the latest
Medical News, keep on logging to Thailand Medical News.
