Duke-NUS scientists in Singapore develop revolutionary blood test for tracking T-cell immunotherapy progress
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 21, 2024 1 year, 3 months, 2 days, 11 hours, 25 minutes ago
Medical News: In a groundbreaking development, scientists from Duke-NUS Medical School in Singapore have created a revolutionary blood test designed to track the progress of T cell-based cancer immunotherapy. This innovative "plug-and-play" test requires only a tiny blood sample to monitor how effectively engineered T cells are functioning in a patient’s body over time. The simplicity of this test could transform how immunotherapies are evaluated and delivered, potentially accelerating advancements in personalized cancer treatment.
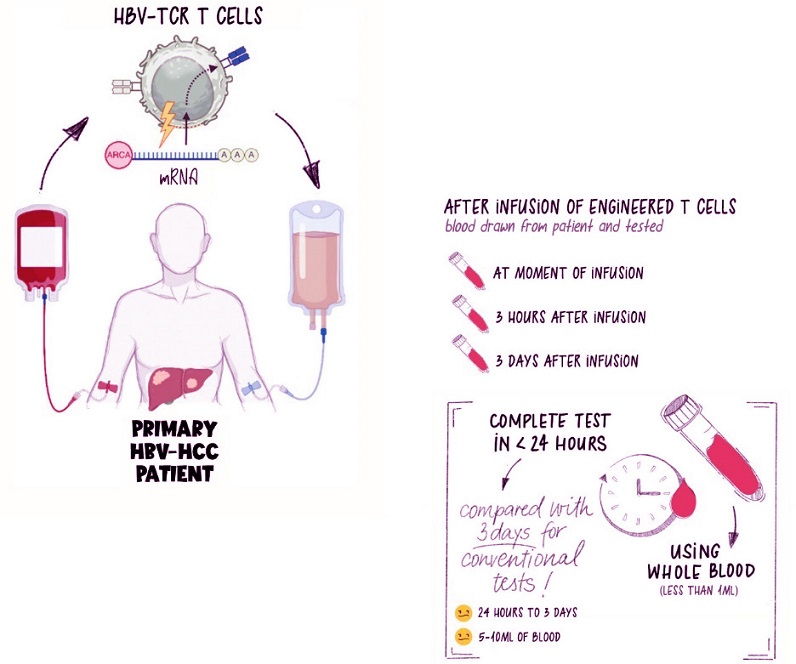 T cells are removed from the patient’s body, engineered to fight disease, then infused back into the patient’s bloodstream. At the point of infusion, and at various time points after, blood is drawn to monitor the efficacy of the T-cell therapy. // Credit: Duke-NUS Medical School
T cells are removed from the patient’s body, engineered to fight disease, then infused back into the patient’s bloodstream. At the point of infusion, and at various time points after, blood is drawn to monitor the efficacy of the T-cell therapy. // Credit: Duke-NUS Medical School
T cells, a crucial component of our immune system, play a vital role in fighting infections and cancer. These cells can be genetically modified to target specific diseases, including cancer, before being reintroduced into a patient’s body. Monitoring their performance has always been a challenge - until now. This
Medical News report delves into how the new test overcomes this obstacle, offering hope for more precise and effective treatments.
How the Test Works
The Duke-NUS team adapted a method initially developed for detecting T cells that respond to SARS-CoV-2, the virus behind COVID-19. This new approach involves stimulating the engineered T cells in a blood sample with peptides (protein fragments) tailored to their target. The test measures the release of cytokines - chemical signals that indicate the T cells are active and functional.
Using less than a quarter of a teaspoon of blood, the test can identify the presence and functionality of engineered T cells in real-time. It’s a quick and efficient solution, allowing clinicians to assess whether these cells are doing their job effectively after being infused into the patient. This ability is critical for ensuring treatments remain effective over time.
Proof of Concept: A Closer Look at the Findings
The team demonstrated the efficacy of their test in a proof-of-concept study published in the peer reviewed journal: Immunotherapy Advances(Oxford Academic).
https://academic.oup.com/immunotherapyadv/article/4/1/ltae007/7759844
They focused on patients receiving immunotherapy for liver cancer caused by the Hepatitis B virus (HBV). By introducing peptides that stimulate HBV-specific T cells, they evaluated whether the engineered T cells retained their functionality after infusion.
The results were promising. The test accurately tracked the presence and performance of T cells over multiple treatment cycles. It also revealed how the infused T cells interacted with the immune system and the tumor environment, providing invaluable insights into their behavior. Importantly, the test detec
ted dose-dependent cytokine release, meaning higher doses of T cells led to more robust immune responses.
A Versatile Tool for Cancer and Beyond
This test is more than just a diagnostic tool - it’s a potential game-changer for immunotherapy research. Its adaptability allows it to be used across various conditions, including viral infections and other types of cancers. The researchers envision its application in tracking both T cell receptor (TCR) and chimeric antigen receptor (CAR) T-cell therapies, which are increasingly being used to treat blood cancers and solid tumors.
In collaboration with Lion TCR Pte Ltd, the test has already been utilized in a clinical trial known as SAFE-T-HBV. This trial assessed a novel HBV-specific TCR T-cell therapy in two patients, showcasing how the test enhances precision in monitoring and improving immunotherapy outcomes.
What Researchers Are Saying
Assistant Professor Anthony Tan, the study's first author, emphasized the test’s simplicity and its potential to make advanced treatments more accessible. “Our innovative test enables us to swiftly detect and analyze engineered T cells in patient blood samples,” he said. “Its simplicity and speed could have a significant impact on the clinical field.”
Senior author Professor Antonio Bertoletti highlighted the test’s broader implications. “Tracking the functionality of adoptively transferred engineered T-cell products could provide important information on treatment efficacy over time,” he noted. “We hope this proof-of-concept will accelerate research into other CAR and TCR T-cell therapies while supporting clinicians caring for patients.”
.jpg) (From left to right) Assistant Professor Anthony Tan, PhD student He Shan and Professor Antonio Bertoletti monitor the effectiveness of T-cell immunotherapy using their newly developed test. // Credit: Duke-NUS Medical School
Future Prospects: Scaling Up the Innovation
(From left to right) Assistant Professor Anthony Tan, PhD student He Shan and Professor Antonio Bertoletti monitor the effectiveness of T-cell immunotherapy using their newly developed test. // Credit: Duke-NUS Medical School
Future Prospects: Scaling Up the Innovation
While the initial results are encouraging, the researchers aim to validate their findings through larger clinical studies. They are optimistic that this test will become a standard tool for tracking engineered T-cell therapies, not only in cancer but also in other diseases where immune system modulation is key.
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, underscored the test’s potential impact. “This innovation isn’t just a step forward in cancer therapy - it’s a significant advancement in patient care,” he stated. “By offering clinicians real-time data on the functionality of these engineered T cells, we are paving the way for highly personalized treatment strategies that could significantly enhance patient outcomes.”
Conclusion: Transforming Cancer Treatment
The development of this novel test represents a major leap forward in the field of immunotherapy. By providing a simple yet powerful tool to monitor the effectiveness of engineered T cells, it addresses a critical gap in cancer treatment. The ability to track T-cell functionality over time ensures that therapies can be tailored to each patient’s needs, improving outcomes and potentially saving lives.
Moreover, its versatility across various applications could accelerate the development of new treatments and vaccines, benefiting countless patients.
The findings from this study highlight the promise of personalized medicine and the potential to transform how we treat cancer and other diseases. The researchers at Duke-NUS, in collaboration with Lion TCR Pte Ltd, have demonstrated the importance of innovation in advancing healthcare. With further clinical trials on the horizon, this test could soon become a cornerstone of modern medicine.
For the latest on Immunotherapy Research, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/singapore-case-study-reveals-that-even-mild-covid-19-can-trigger-fatal-liver-failure-in-patients-with-autoimmune-liver-disease
https://www.thailandmedical.news/news/scientists-discover-a-protein-that-plays-a-key-role-in-regulating-the-body-s-internal-clock
https://www.thailandmedical.news/news/singapore-researchers-discover-how-to-activate-dormant-brain-stem-cells-to-treat-neurodevelopmental-disorders
https://www.thailandmedical.news/news/singapore-study-provides-new-insights-on-how-prior-dengue-infection-influences-covid-19-risk-and-severity
.jpg)