COVID-19 News: New Zealand Study Finds That SARS-CoV-2 Causes Neurons To Degenerate And Die Via Mitochondrial Dysfunction And Disruption Of Metabolism
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 01, 2023 2 years, 3 months, 1 week, 6 days, 8 hours, 3 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has led to unprecedented challenges in healthcare and has prompted extensive research into the diverse array of symptoms and complications associated with the virus. While respiratory symptoms were initially at the forefront of COVID-19 observations, it became apparent that the virus's impact extended far beyond the lungs. In 2020, reports, case studies and
COVID-19 News coverages emerged of neurological symptoms in COVID-19 patients, collectively referred to as NeuroCOVID. These symptoms included dizziness, disturbed consciousness, headache, loss of smell and taste, seizures, encephalitis, and an increased risk of stroke.
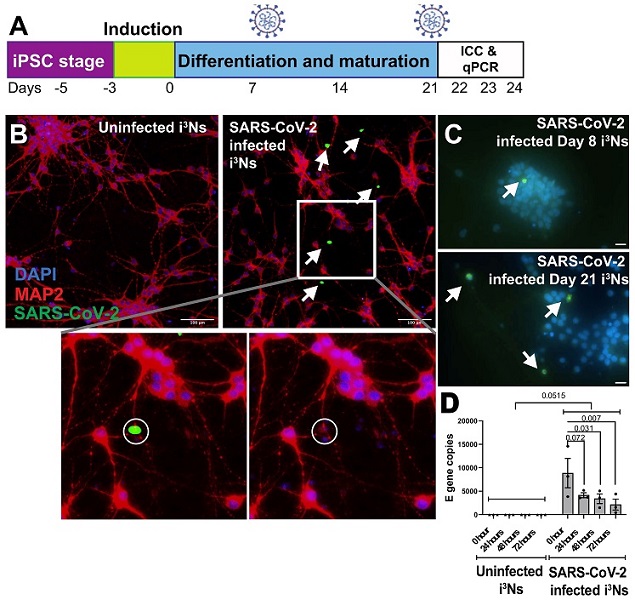 SARS-CoV-2 virus infects human iPSC-derived neurons. (A) iPSCs were differentiated into cortical-like glutamatergic neurons (i3Ns) and infected on Day 21, followed by immunocytochemistry analysis on Day 22. To assess infection in immature i3Ns, infection was carried out on Day 8 and immunocytochemistry on Day 9. Finally, for RT-qPCR analysis of viral replication, i3Ns were infected on Day 21, followed by analysis on Days 22, 23, and 24. (B) i3Ns showed infection 24 h post-infection, as shown by the white arrows. Inset shows that the infected nucleus (DAPI stain) looked fragmented compared to the uninfected nucleus. (C) Day 21 i3Ns showed more SARS-CoV-2 infected cells than Day 8 i3Ns. (D) RT-qPCR analysis of the E gene in infected i3Ns showed no virus replication in the i3Ns.
SARS-CoV-2 virus infects human iPSC-derived neurons. (A) iPSCs were differentiated into cortical-like glutamatergic neurons (i3Ns) and infected on Day 21, followed by immunocytochemistry analysis on Day 22. To assess infection in immature i3Ns, infection was carried out on Day 8 and immunocytochemistry on Day 9. Finally, for RT-qPCR analysis of viral replication, i3Ns were infected on Day 21, followed by analysis on Days 22, 23, and 24. (B) i3Ns showed infection 24 h post-infection, as shown by the white arrows. Inset shows that the infected nucleus (DAPI stain) looked fragmented compared to the uninfected nucleus. (C) Day 21 i3Ns showed more SARS-CoV-2 infected cells than Day 8 i3Ns. (D) RT-qPCR analysis of the E gene in infected i3Ns showed no virus replication in the i3Ns.
Notably, previous experiences with related coronaviruses, such as the 2002 SARS virus, have revealed the presence of viral particles in brain autopsy tissue and spinal cord fluid. Fast forward almost two decades, and a study using transmission electron microscopy on brain sections from a Parkinson's disease patient who contracted SARS-CoV-2 showed the presence of viral particles in the frontal lobe. Additionally, in 2021, a study demonstrated microvascular injuries in the brain and olfactory bulb after SARS-CoV-2 infection.
As the pandemic continued to unfold, researchers sought to understand the virus's impact on the human brain. By 2021, a comprehensive review had documented a wide range of neurological conditions associated with COVID-19 patients, such as gustatory and olfactory dysfunctions, headache, altered mental status, confusion, delirium, dizziness, stroke, cerebral venous thrombosis, seizures, meningoencephalitis, Guillain-Barre syndrome, Miller-Fisher syndrome, acute myelitis, and posterior reversible encephalopathy syndrome. These symptoms affected both adults and children, with even fetal brains showing signs of cortical hemorrhage. Furthermore, studies revealed a higher risk of neurological and psychiatric co-morbidities in patients with severe COVID-19.
https://www.nejm.org/doi/full/10.1056/NEJMc2033369
https://link.springer.com/article/10.1007/s00415-021-10406-y
1648/6695387">https://academic.oup.com/brain/article/146/4/1648/6695387
https://academic.oup.com/brain/article/146/3/1175/6985751
https://www.thelancet.com/journals/lanpsy/article/PIIS2215-03662100084-5/fulltext
In 2022, the UK Biobank reported changes in brain volume in COVID-19 patients, even in those who had recovered, with associated cognitive dysfunction.
https://www.nature.com/articles/s41586-022-04569-5
Despite conflicting studies on whether the virus directly infiltrates the brain, the alterations in brain structure post-SARS-CoV-2 infection suggest a strong link between the virus and neurological symptoms.
A more recent study from September 2022 assessed patients one year after SARS-CoV-2 infection, revealing an increased risk of stroke, cognitive and memory disorders, peripheral nervous system disorders, migraine, seizures, movement disorders, mental health disorders, musculoskeletal disorders, sensory disorders, Guillain–Barre syndrome, encephalitis, or encephalopathy in the post-acute phase of COVID-19.
https://pubmed.ncbi.nlm.nih.gov/36138154/
These findings highlight the importance of long-term follow-up for COVID-19 patients to better understand the virus's enduring effects on the human brain.
Intriguingly, genetic and molecular analyses have indicated that SARS-CoV-2 infection can induce changes in cellular pathways overlapping with neurodegenerative diseases such as Alzheimer's disease, multiple sclerosis, and brain aging. Despite the extensive symptomatic studies and the mounting evidence of SARS-CoV-2's impact on the brain, the underlying molecular mechanisms driving these neurological complications remained inadequately explored.
SARS-CoV-2's Interaction with Human Brain Cells
SARS-CoV-2 enters human cells through various receptors, including ACE-2, TMPRSS2, and TMEM106B. Researchers at the University of Otago, New Zealand, sought to shed light on the molecular mechanisms underlying the neurological symptoms in COVID-19 patients. They employed a unique human-induced pluripotent stem cell-derived neuronal model that expressed ACE-2, TMPRSS2, TMEM106B, and other possible SARS-CoV-2 receptors to evaluate the susceptibility of neurons to SARS-CoV-2 infection. This novel approach involved exposing these neurons to the virus and conducting extensive analyses to uncover the virus's impact on neural cells.
The findings revealed that SARS-CoV-2 infected neurons at a lower rate than other human cells, which was a surprising result given the neurological symptoms observed in COVID-19 patients. However, it was discovered that the virus could not replicate within the neuronal model or produce infectious virions. Despite this, the infected neurons exhibited irregular nuclear morphology compared to other human cells, indicating the presence of cellular changes driven by the virus.
In addition to the direct neuronal infection, researchers also explored the effects of pre-conditioned media from SARS-CoV-2-infected lung cells on neurons. These experiments revealed changes in the neuroproteomic profile of the neurons, particularly affecting mitochondrial proteins and apoptotic and metabolic pathways. These changes provided crucial insights into the mechanisms underlying SARS-CoV-2-mediated neuropathology and its potential contribution to the long-term effects of the virus on the human brain.
Unique Neuroproteomic Changes After SARS-CoV-2 Infection
The study's results confirmed that while SARS-CoV-2 could infect neurons, it did not replicate within them. Instead, infected neurons displayed irregular nuclear morphology and exhibited an upregulation of apoptosis-related proteins. This suggested that the virus's impact on neurons was primarily apoptotic, potentially contributing to the observed neurological symptoms in COVID-19 patients.
Notably, only a limited number of studies have investigated proteomic changes directly in brain cells following SARS-CoV-2 infection, with most focusing on transcriptomic and proteomic changes in body fluids. However, this New Zealand study provided a unique insight into the neuronal proteome alterations associated with SARS-CoV-2 infection.
The study identified several neuroproteomic changes that shed light on the underlying mechanisms of neurological complications in COVID-19 patients. One of the most striking findings was the disruption of mitochondrial proteins, which are essential for energy production and cell survival. The upregulation of mitochondrial proteins suggested that SARS-CoV-2 may affect mitochondrial function in neurons, potentially predisposing them to neurodegenerative diseases such as Alzheimer's.
Furthermore, the study revealed changes in apoptotic pathways, which are related to cell death. The upregulation of apoptotic proteins in infected neurons hinted at the virus's role in driving neural cell death. These findings provided a potential explanation for the neurological symptoms observed in COVID-19 patients, as apoptosis in neurons can lead to a range of neurological complications.
Another critical discovery was the disruption of metabolic pathways in infected neurons, particularly lipid metabolism. While previous studies had shown alterations in host metabolism pathways following SARS-CoV-2 infection, this study was the first to report dysregulated neuronal metabolism, especially in lipid metabolism. The downregulation of proteins related to lipid metabolism in infected neurons highlighted a potential link between disrupted metabolism and the neurological symptoms experienced by COVID-19 patients.
Implications for Future Research
This New Zealand study represents a significant step in understanding the molecular mechanisms underlying the neurological complications associated with COVID-19. While the study focused on neurons, it is essential to recognize that the human brain is a complex network of various cell types, including astrocytes and microglia. These support cells play a crucial role in maintaining neuronal health, and future research should explore the interplay between different brain cell types in the presence of SARS-CoV-2.
Despite its limitations, this study provides valuable insights into the impact of SARS-CoV-2 on the human brain and the potential mechanisms driving neurological symptoms. The findings underscore the importance of continued research into the long-term effects of the virus on the central nervous system and the development of therapies to mitigate its impact on neural health.
In conclusion, the New Zealand study reveals unique neuroproteomic changes in neurons following SARS-CoV-2 infection, offering a potential explanation for the neurological symptoms experienced by COVID-19 patients. This research contributes to the growing body of knowledge surrounding the virus's impact on the human brain and underscores the need for ongoing investigations into the long-term consequences of COVID-19. Understanding the underlying molecular mechanisms is crucial for developing effective therapies and addressing the global health challenges posed by the virus.
The study findings were published in the peer reviewed journal: Biomolecules.
https://www.mdpi.com/2218-273X/13/11/1597#B15-biomolecules-13-01597
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-covid-19-news-italian-study-shows-that-sars-cov-2-infects-dopaminergic-neurons-and-hampers-dopamine-production
https://www.thailandmedical.news/news/breaking-news-french-study-finds-that-sars-cov-2-literally-kills-gnrh-neurons-and-tanycytes,-fueling-lots-of-serious-long-term-health-issues
https://www.thailandmedical.news/news/breaking-covid-19-news-first-study-to-actually-show-and-validate-that-sars-cov-2-can-infect-human-ips-cell-derived-sensory-neurons
https://www.thailandmedical.news/news/breaking-news-study-finds-that-human-ips-cell-derived-sensory-neurons-susceptible-to-sars-cov-2-infection-implications-for-covid-19-neuropathies
https://www.thailandmedical.news/news/university-of-california-study-involving-rhesus-macaques-finds-that-sars-cov-2-virus-infects-the-neurons-of-the-brain-and-induces-neuroinflammation
https://www.thailandmedical.news/news/breaking-multisystem-screening-study-finds-sars-cov-2-proliferation-in-neurons-of-the-myenteric-plexus-and-in-megakaryocytes
https://www.thailandmedical.news/news/breaking-university-of-california-study-alarmingly-confirms-that-sars-cov-2-coronavirus-can-infect-and-replicate-in-human-host-neurons
https://www.thailandmedical.news/news/breaking-university-of-queensland-study-shows-sars-cov-s-fusogens-cause-host-neurons-and-glial-cells-to-fuse-resulting-in-neurological-issues
