SARS-CoV-2 Destroys Heart Cell Mitochondria Leading to Dangerous Long COVID Cardiac Issues
Nikhil Prasad Fact checked by:Thailand Medical News Team May 11, 2025 9 months, 1 week, 5 days, 19 hours, 4 minutes ago
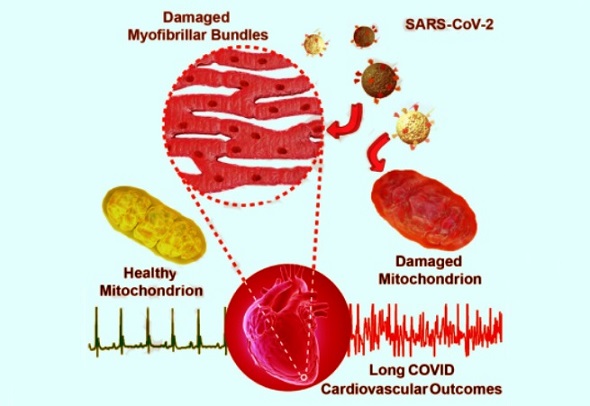
Medical News: In a groundbreaking study conducted by top medical scientists in China, alarming evidence has emerged showing that SARS-CoV-2, the virus responsible for COVID-19, severely damages the mitochondria in heart muscle cells — even long after the infection has passed. This could explain the growing number of people suffering from ongoing heart problems months after recovering from COVID-19.
 SARS-CoV-2 Destroys Heart Cell Mitochondria Leading to Dangerous Long COVID Cardiac Issues
SARS-CoV-2 Destroys Heart Cell Mitochondria Leading to Dangerous Long COVID Cardiac Issues
The research was carried out by experts from several leading institutions, including the Department of Cardiology at Shanghai Tenth People’s Hospital of Tongji University School of Medicine, the Cryo-electron Microscopy Center and the School of Life Science at Southern University of Science and Technology in Shenzhen, the Guangzhou Institute of Respiratory Health at Guangzhou Medical University, the School of Medical Technology at Beijing Institute of Technology, and Thermo Fisher Scientific’s Shanghai NanoPort. This
Medical News report reveals deeply concerning findings from both human patients and laboratory mice, shining new light on how COVID-19 may be triggering long-term cardiovascular damage.
Five Real Life Cases Show Mitochondrial Damage in Heart Cells
The study began with close examination of five patients who had previously recovered from COVID-19 but later developed serious heart conditions. These conditions included sudden cardiac arrest, myocarditis, and chest pain. Researchers performed endomyocardial biopsies — an invasive but gold-standard method to study heart tissue at a microscopic level — and used advanced electron microscopy techniques to examine the tissue.
In all five patients, scientists observed widespread destruction of the mitochondria inside cardiomyocytes, which are the cells responsible for keeping the heart beating. Mitochondria are often referred to as the power plants of cells, as they generate the energy needed for the heart to function. In these patients, the mitochondria were swollen, vacuolated (filled with empty spaces), and showed broken internal structures called cristae. Some cells also had signs of fibrosis and lipofuscin accumulation, indicating cell aging and damage.
Strikingly, these mitochondrial abnormalities persisted even 76 to 85 days after the patients had recovered from COVID-19, suggesting that the virus may have lasting effects long after it disappears from the body.
Animal Experiments Confirm COVID-19 Directly Harms Heart Cells
To confirm their findings, the researchers infected laboratory mice with Omicron subvariants BA.5.2 and BQ.1 of SARS-CoV-2. These mice, although not exhibiting serious symptoms, developed the same kind of mitochondrial damage in their heart tissues that was seen in the human patients. Again, mitochondrial swelling, cristae fragmentation, and myofibril disorganization were observed.
This strongly indicates that the SARS-CoV-2 virus itself — rather than immune complications or other unrela
ted health problems — is directly responsible for the heart cell damage. The scientists did not find active virus in the heart tissue after infection, which means the damage could be a lingering effect of earlier viral invasion.
Advanced Imaging Reveals Alarming Levels of Mitochondrial Breakdown
Using state-of-the-art imaging tools like focused ion beam scanning electron microscopy (FIB-SEM) and artificial intelligence-powered segmentation software, the researchers created detailed 3D models of damaged mitochondria. They then measured “cristae density” — a marker of how intact the internal energy-producing structures of mitochondria are.
In healthy mitochondria, this value was around 28–32 micrometers, but in damaged mitochondria from COVID-19 patients and infected mice, it dropped to as low as 10. This drastic loss of structure likely means these cells can no longer produce energy efficiently, setting the stage for heart problems like arrhythmias, fatigue, and chest pain.
Even more shocking, when analyzing large 3D volumes of tissue, the proportion of mitochondria classified as severely disordered reached up to 90% in some cases.
Proteomics Analysis Links Damage to Disruption of Key Mitochondrial Proteins
The research didn’t stop at imaging. Using a powerful protein analysis method called quantitative mass spectrometry, the team discovered that SARS-CoV-2 infection caused major shifts in proteins related to mitochondrial function. Key proteins responsible for transporting molecules into mitochondria (such as TOM40, TOM20, and TIM13), maintaining mitochondrial shape (like Samm50 and MPV17), and producing cellular energy (including NADH dehydrogenase and cytochrome c oxidase) were significantly altered.
Some of these proteins were upregulated, likely as a response to mitochondrial stress and damage. Others, such as TOM70, which is critical for mitochondrial health, have been shown in past studies to be suppressed by SARS-CoV-2 viral proteins.
This explains how the virus can trigger a cascade of molecular disruptions inside the heart, even after the acute infection phase has ended.
Long COVID Heart Symptoms May Be Due to Ongoing Energy Crisis in Cells
The study’s findings help to explain why so many people who had even mild COVID-19 go on to experience chest tightness, heart palpitations, or exercise intolerance — classic symptoms of long COVID. Mitochondrial dysfunction prevents heart cells from generating the energy needed for proper contractions, leading to irregular heartbeats and overall cardiovascular weakness.
Even though only five patients were studied, the findings are consistent with mounting reports that long COVID is not merely a psychological or vague condition — it causes real, measurable damage at the cellular level.
Takeaways and Implications
This detailed investigation shows that SARS-CoV-2 can cause lingering, possibly irreversible damage to heart cell mitochondria. Even months after the infection, heart tissue still shows signs of decay and dysfunction, suggesting that the heart is still recovering from an energy production crisis. While the virus may no longer be present, the body continues to suffer its consequences. These insights should influence future recommendations regarding return to exercise after COVID-19, especially for athletes and individuals experiencing post-COVID symptoms.
Moreover, the research emphasizes the urgent need for long-term cardiovascular monitoring in COVID-19 survivors and suggests that healthcare providers should be alert for hidden heart damage in patients even if they appear to have recovered from the infection.
The study findings were published in the peer reviewed Journal of Advanced Research.
https://www.sciencedirect.com/science/article/pii/S2090123225003066
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/viruses-including-sars-cov-2-silently-attack-heart-communication-proteins-leading-to-deadly-arrhythmias-and-myocarditis
https://www.thailandmedical.news/news/breaking-sars-cov-2-encodes-circular-rnas-that-can-impair-endothelial-cells-and-cause-cardiovascular-issues
https://www.thailandmedical.news/news/american-study-discovers-that-covid-19-alters-heart-glycosylation-leading-to-potential-cardiac-complications
https://www.thailandmedical.news/articles/coronavirus
https://www.thailandmedical.news/pages/thailand_doctors_listings
