COVID-19 News: Italian Study Finds That SARS-CoV-2 Infections Causes Alterations Of Circulating Peptidome In Human Host!
COVID-19 News - Alterations Of Circulating Peptidome Jan 23, 2023 3 years, 3 weeks, 1 day, 2 hours, 53 minutes ago
COVID-19 News: A new study by researchers from the University of Piemonte Orientale – Italy and Sant’Andrea Hospital – Italy has found that SARS-CoV-2 infections leads to alterations of the circulating peptidome.
Circulating peptides are involved and play a critical role in many cellular and physiological functions and passively diffuse through cell membranes and epithelial barriers. Any alterations or modulation to these circulating peptides can also result in serious immediate or even long-term health consequences.
Although the impact of COVID-19 on the host proteome, metabolome, and lipidome has been largely investigated and covered in various past
COVID-19 News coverages, the circulating peptidome remains unexplored.
The study team are the first to apply an untargeted peptidomic approach to provide insight into alterations of circulating peptides in the development and severity of SARS-CoV-2 infection.
The circulating peptidome from COVID-19 severe and mildly symptomatic patients and negative controls was characterized utilizing LC-MS/MS analysis for identification and quantification purposes. Database search and statistical analysis allowed a complete characterization of the plasma peptidome and the detection of the most significant modulated peptides that were impacted by the infection.
The study findings showed that peptidomic alterations were mostly seen in peptides derived from fibrinogen alpha chain (FIBA_HUMAN), albumin (ALBU_HUMAN), apolipoprotein A-II (APOA2_HUMAN), and haptoglobin (HPT_HUMAN).
The study findings highlighted not only that peptide abundance inversely correlates with disease severity, but also the involvement of biomolecules belonging to inflammatory, immune-response, and coagulation proteins/processes.
Alarmingly, the study findings also suggested a possible involvement of changes in protein degradation patterns.
The study findings also enabled the identification of circulating peptides potentially playing a crucial role in the progression of COVID-19.
The study findings were published in the peer reviewed International Journal of Environmental Research and Public Health.
https://www.mdpi.com/1660-4601/20/2/1564
Interestingly, the comparison between the peptidome profile of SARS-CoV-2 positive and negative patients reported the quantitative modulation of 73 peptides from 31 different proteins, the majority of which were downregulated in COVID-19 infected patients (fold change < 0.5). Of those peptides, 14 were from albumin and their decrease is in line with the well-documented decrease in albumin levels in COVID-19 patients.
https://www.degruyter.com/document/doi/10.1515/cclm-2020-0369/html
The decrease of apolipoprotein A-1 derived peptides (10 peptides) is in accordance with the reported decrease of circulating HDL.
https://pubs.ac
s.org/doi/full/10.1021/acs.jproteome.0c00519
For apolipoprotein C-III the total levels were reported to be dysregulated in relation to COVID-19, confirming the observed changes in the degradation pattern with an increase of cutting efficiency at position 28 that justifies the increase of S21EAEDASL28 peptide, and the concomitant decrease of 21–84 and 21–34 fragments upon infection.
https://www.sciencedirect.com/science/article/pii/S2405471221001605
https://www.mcponline.org/article/S1535-9476(20)35154-9/fulltext
The study findings also showed that a number of peptides deriving from proteins associated with the inflammatory process are decreased in SARS-CoV-2 infected patients: for example, human alpha-1-antitrypsin (A1AT_HUMAN), human inter-alpha-trypsin inhibitor heavy chain H2 (ITIH2_HUMAN), human complement C3 (CO3_HUMAN), and human complement C1q subcomponent subunit B (C1QB_HUMAN) peptides.
Strangely, although alpha-1-antitrypsin and inter-alpha-trypsin inhibitors are reported to increase during COVID-19, the derived peptides are decreasing in this study analysis (5 concordant peptides for both).
https://pubmed.ncbi.nlm.nih.gov/34367265/
This may indicate enhanced stability or escape from degradation may contribute to the increase of circulating levels.
A1AT or human alpha-1-antitrypsin is a circulating protease inhibitor mainly synthesized by hepatocytes and targeting neutrophil elastase, whose activity is critical for the prevention of proteolytic tissue (lung and liver) damage.
https://pubmed.ncbi.nlm.nih.gov/16284217/
A dual role of A1AT has been discovered, both as an antiviral and anti-inflammation protein. Indeed, alpha-1-antitrypsin not only can protect the lung from damages such as emphysema by inhibiting neutrophilic elastase (anti-inflammation role) but can also block SARS-CoV-2 infection through the inhibition of a protease involved in the entry of SARS-CoV-2 into the host cells (transmembrane protease serine-type 2-TMPRSS2), thus exerting an antiviral action.
https://pubmed.ncbi.nlm.nih.gov/33362565/
https://www.nature.com/articles/s41467-021-21972-0
Immune dysregulation and the more severe symptoms shown by COVID-19 patients may be so associated with alterations in A1AT levels.
Similarly, inter-alpha-trypsin inhibitors are an acute phase protein family with matrix protective activity through protease inhibitory action. Since previous studies pointed out that low levels of inter-alpha-trypsin inhibitor proteins correlated with severe sepsis and influenced patient survival, these observations may help explain the downregulation of human inter-alpha-trypsin inhibitor heavy chain H2 peptide in SARS-CoV-2 infected subjects, when compared to negative controls.
Importantly, the complement system fragments were under-expressed in positive subjects. As diffuse complement activation is one of the key features of COVID-19, a variation of complement factors derived peptides is not surprising.
The study findings showed that complement C1q and C3 fragments (respectively, 1 and 6 peptides) decreasing during infection, and C4 changing digestion pattern with 1 peptide downregulated and 3 upregulated. Even if the involvement of the humoral immune response in COVID-19 development has been largely demonstrated, the role of various complement system components in the prognosis of SARS-CoV-2 infection remains nevertheless unclear.
A past study that investigated the clinical and immunological characteristics of COVID-19 patients stratified on the basis of disease severity, found that complement C1q subcomponent subunit B levels were, for instance, significantly reduced in severe cases. Conversely, complement C3 levels have been either associated with poor prognosis in severe patients or addressed as not predictive of disease progression. It can be assumed that the alterations in the degradation pattern of the considered proteins correlate with the hyperactivation of the complement pathway already demonstrated in SARS-CoV-2 infection.
https://journals.asm.org/doi/full/10.1128/mSphere.00362-20
https://www.sciencedirect.com/science/article/pii/S1567576920316970
https://www.frontiersin.org/articles/10.3389/fimmu.2021.696085/full?fbclid=IwAR1RkonAd7iCVE_T4oGn0OgLAa2Mgz1bL1Ye-avsPAQ-jlwak9-hbg6WtN4
https://link.springer.com/article/10.1007/s12250-020-00203-8
In contrast, the comparison of COVID-19 positive subjects vs. negative controls also established the upregulation of only a few peptides: more specifically, transthyretin (TTHY_HUMAN), isoform 2 of Haptoglobin (HPT_HUMAN), Ceruloplasmin (CERU_HUMAN), isoform 2 of Fibrinogen alpha chain (FIBA_HUMAN), and isoform 2 of Complement C4-A (CO4A_HUMAN) peptides.
Transthyretin is an acute phase-reactant acting as a hormone transporter whose levels negatively correlate with inflammation, so much that low concentrations of TTHY are indicative of a systemic inflammatory state.
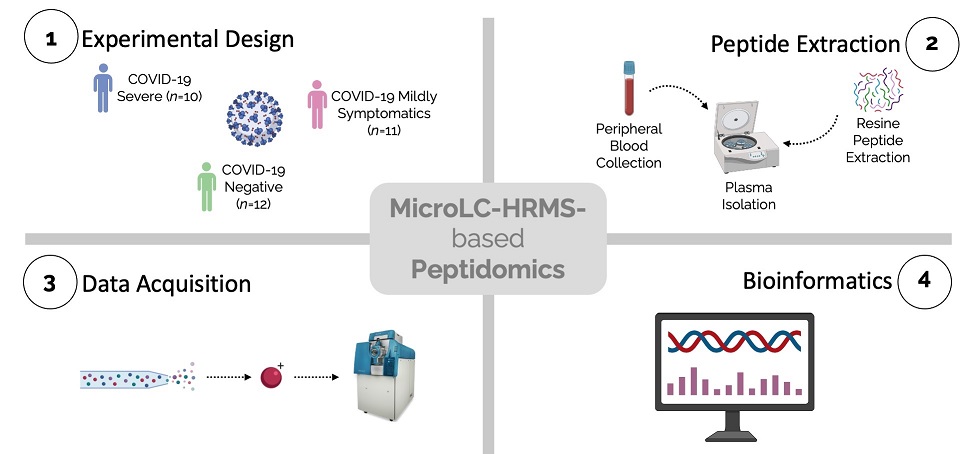 Graphical Abstract
Graphical Abstract
The study findings highlight a decrease in transthyretin protein levels and suggest a parallel change in degradation with an increase cutting at position 137 indicated by increased S137TTAVVTNPKE147 and the concomitant decrease of 129–147, 130–147, and 116–147 fragments. Although transthyretin protein has been proposed as a marker of nutritional status, the potential contribution of the feeding status in the modulation of identified peptides was excluded.
Significantly, TTHY is a marker of malnutrition and of prognosis associated with malnutrition, and, although dietary questionnaires were not available, no malnourished patients were reported in the clinical data. In addition, all the samples were obtained at the diagnosis of the infection, as soon as the patients were recovered at the hospital, thus excluding any impact of the therapy and the disease on nutrition. However, further studies are needed to definitely exclude the contribution of feeding status.
It was also noted that severe COVID-19 patients often exhibit signs of hemolysis and consequently elevated levels of free heme, whose excess promotes oxidative and inflammatory stress. The acute phase protein haptoglobin (Hpt) can scavenge free heme, preventing heme-induced inflammation, but in the case of severe hemolysis, this protein may be overwhelmed, hence not completely neutralizing heme’s dangerous effects.
https://www.mdpi.com/2076-3921/9/6/540
Hence, the observed upregulation of HPT_HUMAN peptide in mild to severe SARS-CoV-2 patients, led the study team to postulate that infected subjects show high levels of the related protein, which is unable to efficiently neutralize free heme. Moreover, the presence of an upregulated peptide together with two downregulated ones strongly suggests a change in the degradation pattern.
Ceruloplasmin (Cp), positive acute phase reactant, and the coagulation factor fibrinogen are known key molecular players in inflammation. For this reason, it is not surprising to notice an increase in the corresponding peptide levels and of the whole proteins as well in COVID-19 positive patients.
The modulation of complement C4-A peptide in SARS-CoV-2 infected subjects is consistent with the changes in complement pathway activation stated before.
The lists of severe to negative and mild to negative peptides significantly overlap with the previous data, with the interesting exception of a peptide derived from the cytoskeletal protein talin. This peptide is specific for severe patients and one of the features discriminating mild to severe ones. Other differences are instead quantitative, with a stronger upregulation of transthyretin, modulation of haptoglobin peptides, and downregulation of fibrinogen, complement, and alpha-1-antitrypsin in severe versus mild cases.
The broad spectrum of the COVID-19 symptoms seems to be attributable to a variety of pathophysiological mechanisms. Among them, matrix metalloproteinases (MMPs), a family of zinc-dependent and Ca2+-containing endoproteinases deriving from inflammatory and parenchymal cells in the lung, have been already reported as involved in pulmonary pathologies characterized by tissue remodeling and acute lung injury (e.g., asthma, COPD).
https://www.mdpi.com/2218-273X/11/3/390
The study team noted that despite the involvement of protease activity in COVID-19, it is not easy to link peptide abundance to changes in the activity of specific proteases.
A detailed search in the MEROPS protease database indicates that the proteases responsible for the generation of the above-mentioned peptides are largely unknown.
https://academic.oup.com/nar/article/46/D1/D624/4626772
A prediction based on the cleavage sites indicates the metalloproteinase family, catepsin family, meprin A, pepsin A-3, and calpain1/2 as proteases generating the regulated peptides. Changes in protease activity are indeed expected in an inflammatory disease with strong neutrophil and complement activation. In particular, a strong increase in matrix-metalloproteinase 9 (MMP-9), whose correlation with acute lung injury and chronic respiratory diseases is a fact, has been reported in COVID-19 patients and associated with respiratory failure.
https://pubmed.ncbi.nlm.nih.gov/32603675/
A correlation between MMP-3 serum levels and the severity of COVID-19 pulmonary symptoms has been also assessed. Thus, given the observed alterations in the degradation pattern of the cited proteins and the reported involvement of metallopeptidases in SARS-CoV-2 infection, there may be a link between these two phenomena impacting COVID-19 severity. However, it must be noted that the changes in the circulating peptidome observed are the result of a complex balance between target abundance, protease activity, and clearance rate, all of which could be modulated by COVID-19.
The study findings showed that peptides’ abundances are correlated not only with the infection but also with the severity of the disease. The untargeted quantitative mapping of those peptides showed their derivation from proteins related to inflammation, coagulation, and immune responses.
These three biological pathways are well-known players in COVID-19 disease. In addition, comparing the study data with the published literature, the study team found that several peptides did not reflect the originating protein abundances, suggesting the presence of other biochemical mechanisms linked to peptide releases and/or uptake by cells.
The observed change in peptidome composition diverges from those previously reported by other inflammatory conditions such as in septic shock patients where induction of protease activity and an increase of peptide abundance correlate with disease severity.
https://pubmed.ncbi.nlm.nih.gov/30336851/
To date this is the only study that investigated how SARS-CoV-2 infection affects circulating peptides, a larger cohort of patients, including the asymptomatic ones, would allow the identification of more specific pathways and peptides associated with the development of the disease. A larger study with greater statistical power would also help to overcome high inter-variability between subjects.
More detailed studies would also show the long-term health implications of these changes in circulating peptides in infected human host.
For the latest
COVID-19 News, keep on logging to Thailand Medical News.

