BREAKING! University Of Sydney Study Discovers A New Receptor LRRC15 That Binds With SARS-CoV-2 And Suppresses Infection And Regulates Fibrosis
Source: Medical News-LRRC15 And SARS-CoV-2 Nov 17, 2021 4 years, 2 months, 3 weeks, 6 days, 5 minutes ago
A new study led by researchers from the University of Sydney and aided by a specialist from the University of New South Wales (Both of which are the only two leading credible medical and biological sciences research entities in the state of New South Wales, Australia) have in a new study discovered a new receptor called
LRRC15 that the SARS-CoV-2 spike proteins are able to bind with and that also suppresses infection while regulating lung fibrosis.

Though the ACE2 receptors are the primary receptors for SARS-CoV-2 infection, a systematic assessment of factors controlling SARS-CoV-2 host interactions has not been described.
The study team utilized whole genome CRISPR activation to identify host factors controlling SARS-CoV-2 Spike binding.
Interestingly the top result was a Toll-like receptor-related cell surface receptor called leucine-rich repeat-containing protein 15 (LRRC15).
The study findings showed that LRRC15 expression was sufficient to promote SARS-CoV-2 Spike binding where it forms a cell surface complex with LRRC15 but does not support infection. Instead, LRRC15 functioned as a negative receptor suppressing both pseudotyped and live SARS-CoV-2 infection.
Importantly, LRRC15 is expressed in collagen-producing lung myofibroblasts where it can sequester virus and reduce infection in trans. Mechanistically LRRC15 is regulated by TGF-β, where moderate LRRC15 expression drives collagen production but high levels suppress it, revealing a novel lung fibrosis feedback circuit. Overall, LRRC15 is a master regulator of SARS-CoV-2, suppressing infection and controlling collagen production associated with “long-haul” COVID-19.
.jpg) (A) Luciferase assay for quantification of SARS-CoV-2 pseudovirus infection in (B) WT HEK293T (n=4) and (C) HEK293T-ACE2-TMPRSS2(n=3). Cells were transfected with plasmid encoding LRRC15 transcript 1 or empty vector as a control. Luminescence for LRRC15 cells were normalized to Control cells. Significance was determined by two-way ANOVA, Sidak multiple comparison test; ****p<0.0001,***p<0.001,**p<0.01,*p<0.05. (D) Cell death assay for quantification of D614G SARS-CoV-2 live virus infection in HEK293T-ACE2-TMPRSS2 cells. Cell death was determined via nuclei counts 48 hours after addition of virus. (E,F) Quantification of cell survival after incubation with (E) D614G (n=4) and (F) Delta (n=3) SARS-CoV-2 live virus. Significance was determined by two-way ANOVA, *p<0.05.
(A) Luciferase assay for quantification of SARS-CoV-2 pseudovirus infection in (B) WT HEK293T (n=4) and (C) HEK293T-ACE2-TMPRSS2(n=3). Cells were transfected with plasmid encoding LRRC15 transcript 1 or empty vector as a control. Luminescence for LRRC15 cells were normalized to Control cells. Significance was determined by two-way ANOVA, Sidak multiple comparison test; ****p<0.0001,***p<0.001,**p<0.01,*p<0.05. (D) Cell death assay for quantification of D614G SARS-CoV-2 live virus infection in HEK293T-ACE2-TMPRSS2 cells. Cell death was determined via nuclei counts 48 hours after addition of virus. (E,F) Quantification of cell survival after incubation with (E) D614G (n=4) and (F) Delta (n=3) SARS-CoV-2 live virus. Significance was determined by two-way ANOVA, *p<0.05.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.11.09.467981v1.full
Thailand Medical news would like to add that an earlier study
conducted by British and German researchers also identified LRRC15 as possible host receptors for the SARS-CoV-2 spike proteins and also their possible role in mediating infection.
https://www.thailandmedical.news/news/breaking-uk-and-german-scientists-discover-that-protein-lrrc15-interaction-with-sars-cov-2-spike-protein-could-be-modulating-infection
The COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus has been one of the greatest public health challenges worldwide. The World Health Organization (WHO) reports more than 255 million cases of SARS-CoV-2 infection and over 5.12 million deaths since the beginning of the pandemic.
Both the SARS-CoV-1 and SARS-CoV-2 coronaviruses have similar sequences, with their shared primary entry receptor being the angiotensin-converting enzyme 2 (ACE2) receptor. Several studies regarding other host factors that can influence the spike binding of SARS-CoV-2 are limited.
The Australian study team conducted a research to identify novel host factors affecting the cellular interactions of the SARS-CoV-2 spike protein using whole-genome clustered regularly spaced short palindromic repeats (CRISPR) activation.
Surprisingly a toll-like receptor (TLR)-related cell surface receptor known as leucine-rich repeat-containing protein 15 (LRRC15) was identified in three whole-genome screens as a promoter of SARS-CoV-2 spike binding.
Detailed flow cytometry, immunoprecipitation, and confocal microscopy confirmed that LRRC15 forms a cell surface complex with the virus but did not support infection.
It should be noted that LRRC15 is not an entry receptor for SARS-CoV-2; however, its ectopic expression inhibits SARS-CoV-2 pseudovirus infection and reduces live SARS-CoV-2 infection.
Previous studies have found ACE-2 as the primary receptor for the SARS-CoV-2 spike protein. The research team of the current study used CRISPR activation screening to identify other host factors influencing the spike488 protein interaction.
Interestingly (cDNA) HEK293TACE2 has a high affinity to spike488 as compared to wild-type HEK293T cells. Later, three single guide ribonucleic acid (sgRNA) clones of HEK293T-CRISPRa also showed similar results as cDNA overexpression and confirmed that CRISPRa induced ACE2 expression benefits spike488 binding.
The study team collected genomic DNA from unselected or spike488-selected cells and the abundance of sgRNA was quantified by sequencing. The data were subsequently analyzed using the MAGeCK analysis platform and plotted with MAGeCKFlute.
Surprisingly transmembrane protein LRRC15 was a top hit at an FDR cut-off of 0.25, followed by the SARS-CoV-2 entry receptor ACE2.
In order to confirm the role of LRRC15 in SARS-CoV-2 spike binding, the study team transfected LRRC15-GFP cDNA into HEK293T cells and analyzed spike647 binding through flow cytometry.
The study findings showed that LRRC15 was found to have two isoforms of LRRC15_1 and LRRC15_2, both of which showed strong spike binding. No spike647 binding was observed in cells transfected with a green fluorescent protein (GFP).
The findings also showed that LRRC15 expression promotes spike binding in HEK293T-ACE2-TMPRSS2 cells and that both isoforms of LRRC15 colocalized with spike647.
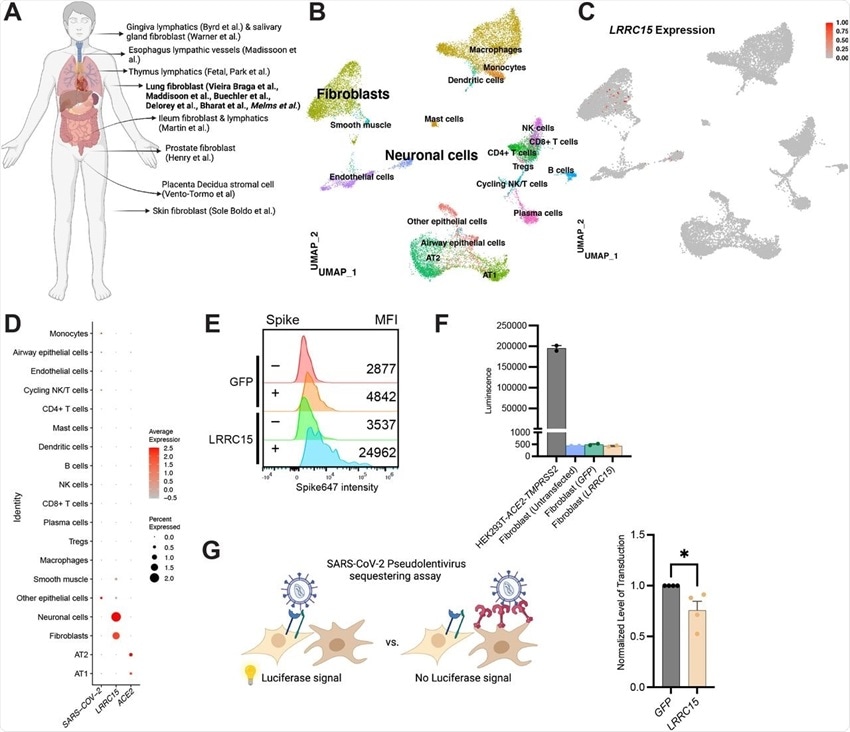 (A) Overview of cell types expressing LRRC15 from existing single cell RNA-sequencing datasets.
(A) Overview of cell types expressing LRRC15 from existing single cell RNA-sequencing datasets.
(B) UMAP plot of lung single nucleus RNAseq dataset (Melms et al).
(C) Feature plot and (D) Dotplot shows LRRC15 is expressed in fibroblasts and neuronal cells. Expression of LRRC15 in fibroblasts is also observed in fibroblasts of separate studies.
(E) Fibroblasts have intrinsic spike binding ability that can be further enhanced by LRRC15 overexpression. Fibroblasts were transfected with empty vector control or LRRC15 cDNA, and spike binding capacity was quantified via flow cytometry. MFI = Mean Fluorescence Intensity.
(F) Fibroblasts do not have innate tropism for SARS-CoV-2 and overexpression of LRRC15 does not mediate infection. Untransfected, GFP and LRRC15-GFP transfected fibroblasts were transduced with 5×108SARS-CoV-2 pseudovirus particles for 24 hours before quantification via luciferase assay. Transduction efficiency (luciferase luminescence) was compared to permissive cell line HEK293T-ACE2-TMPRSS2.
(G) LRRC15 expressing fibroblasts reduced SARS-CoV-2 pseudovirus transduction in HEK293T-ACE2-TMPRSS2. Luminescence of LRRC15+ co-culture was normalized to control GFP co-culture, and significance was determined by Mann-Whitney One-tailed test, *p<0.05.
In oder to confirm the interaction between the SARS-CoV-2 spike protein and LRRC15, the spike was added to LRRC15-expressing cells, followed by the immunoprecipitation of LRRC15.
In the case of both LRRC15_1 or LRRC15_2, the spike protein was co-immunoprecipitated in the eluate.
When taken together, these study findings demonstrate that expression of LRRC15 can promote SARS-CoV-2 spike binding to HEK293T cells, where LRRC15 enhances the spike interactions in the presence of ACE2 and TMPRSS2.
The study team also evaluated the role of LRRC15 as an entry receptor for SARS-CoV-2 and revealed that it does not serve as an entry receptor; however, LRRC15 can suppress spike-mediated entry and infection of SARS-CoV-2.
The study also used the COVID-19 Cell Atlas data set to confirm LRRC15 expression in multiple lymphatic vessels, placenta decidua stromal cells, and fibroblasts from the skin, prostate, and lungs.
Significantly, LRRC15 expression was subsequently confirmed in lung fibroblasts and neuronal cells in non-SARS-CoV-2-infected individuals.
The similar results were observed in other COVID-19 patients’ single-cell RNA sequencing data.
These study findings support the study team’s in vitro observations, which show that LRRC15 acts as an innate immune barrier and does not facilitate SARS-CoV-2 infection.
Just as important, In vitro studies of COVID-19 patients’ lungs showed that collagen-producing fibroblasts express LRRC15, which may regulate lung fibrosis.
Hence based on all these findings, the study team concluded that the TLR-related receptor LRRC15 is a regulator of infection as well as fibrosis, thus controlling the outcomes of SARS-CoV-2 infection and “long-haul” COVID-19.
Corresponding author Professor Dr G. Gregory Neely from the Charles Perkins Centre, Dr. John and Anne Chong Lab for Functional Genomics, Centenary Institute, and School of Life and Environmental Sciences, University of Sydney told Thailand
Medical News, “While our data highlights a new role for LRRC15 in promoting SARS-CoV-2 Spike binding, limiting infection, and regulating collagen expression, it is currently unclear how LRRC15 contributes to human COVID-19 disease. We consider multiple possible mechanisms. In vivo, LRRC15 may provide an innate barrier that can slow infection or limit transmission, allowing additional innate mechanisms to clear SARS-CoV-2. For example, LRRC15 could mediate a Tetherin-like function to anchor exiting viral particles to limit spread of the virus through the tissue. Since in the lung LRRC15 is found in ACE2 negative cells, LRRC15 could primarily act to physically sequester SARS-CoV-2 virions away from permissive cells. Alternatively, the level of LRRC15 expression could control how lung or other tissue fibroblasts react to infection. We found LRRC15 is regulated by TGFβ, and others have reported LRRC15 is upregulated by proinflammatory cytokines including TNFα, IL-1β and IFNγ. Thus, under conditions of appropriate inflammation, LRRC15 may be upregulated and play a primary role in immobilizing and sequestering viral particles to control infection while also suppressing lung fibrosis. LRRC15 may even help fibroblasts pass immobilized virus to innate lung antigen presenting cells, and a recently published spatial-resolution single cell analysis of the lung in COVID-19 showed that lung fibroblasts interact with SARS-CoV-2 Spike+ macrophages and dendritic cells. Then when inflammation subsides, LRRC15 levels decrease, and lower levels of LRRC15 then promote collagen deposition supporting lung repair. When this system is dysregulated, for example in conditions of chronic lung infection, LRRC15 levels may drop during infection, with inappropriate collagen production then leading to ‘long- haul’ COVID.”
He added, “Further investigation into how LRRC15 contributes to SARS-CoV-2 pathology will help us better understand and treat this and future pandemics.”
For the latest
SARS-CoV-2 Research, keep on logging t

.jpg)
