BREAKING NEWS! Study Shows That Asymptomatic COVID-19 Infections End Up With Sub-Clinical Vascular Damage And Red Blood Cell Turnover!
TMN News Team Aug 02, 2023 2 years, 6 months, 2 weeks, 2 days, 21 hours, 1 minute ago
Asymptomatic COVID-19 individuals display elevated vascular endothelial cell turnover and erythropoiesis according to epigenetic liquid biopsies!
COVID-19 News: In the midst of the ongoing COVID-19 pandemic, scientists at the Hebrew University of Jerusalem have made a groundbreaking discovery that sheds new light on the intricate mechanisms behind SARS-CoV-2 infection. Their study, which involved the analysis of DNA methylation and histone modification patterns in circulating chromatin, has revealed surprising findings about the impact of the virus on human tissues.
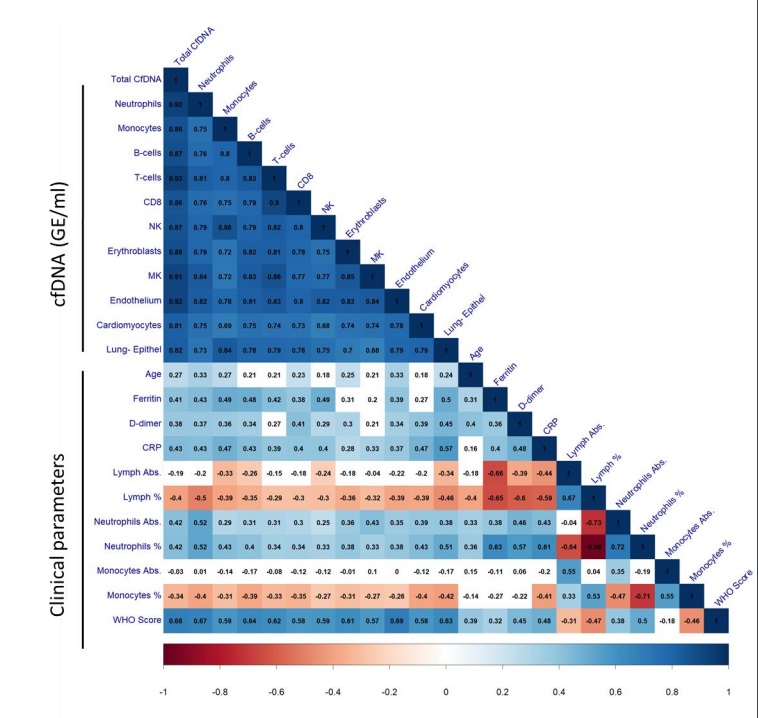 Correlations between cell type-specific cfDNA, clinical and laboratory parameters, and WHO clinical score. Correlation coefficients are presented in black inside each square. Only statistically significant correlations (q<0.05) are colored, and the blue-white-red color scale reflects of coefficient value.
Correlations between cell type-specific cfDNA, clinical and laboratory parameters, and WHO clinical score. Correlation coefficients are presented in black inside each square. Only statistically significant correlations (q<0.05) are colored, and the blue-white-red color scale reflects of coefficient value.
Previous
COVID-19 News coverages had already showed that even asymptomatic sars-cov-2 infections cause DNA methylation and epigenetic changes, leading to immune dysregulation!
https://www.thailandmedical.news/news/breaking-news-even-asymptomatic-sars-cov-2-infections-cause-dna-methylation-and-epigenetic-changes,-leading-to-immune-dysregulation
SARS-CoV-2, the virus responsible for COVID-19, has been known to cause damage to various organs, such as the lungs, heart, blood vessels, pancreas, kidneys, and liver. However, the full spectrum of tissues affected by the virus has remained largely elusive, and understanding this is crucial for deciphering the varied clinical course of the disease, ranging from severe symptoms to asymptomatic presentations.
The research team conducted an extensive analysis of cell-free DNA (cfDNA) levels in severe and asymptomatic COVID-19 patients. Their findings were astonishing, indicating elevated turnover and cell death in multiple tissues, including lung epithelial cells, cardiomyocytes, vascular endothelial cells, and erythroblasts (immature red blood cells).
Of particular significance, the study showed that asymptomatic patients, who show no outward signs of damage to the lungs or heart, displayed elevated levels of vascular endothelial cell and erythroblast cfDNA. This suggests that sub-clinical vascular and erythrocyte turnover are universal features of COVID-19, irrespective of disease severity.
Liquid biopsies, which involve the analysis of cfDNA methylation and chromatin patterns, provided critical insights into the dynamics of the disease. This non-invasive technique allowed researchers to assess cellular turnover, cell death, and gene expression programs operating in cells prior to their demise.
The correlation between disease severity and various cfDNA parameters, including immune cell types and solid organs, was stronger than the correlation to standard bi
omarkers such as CRP, ferritin, d-dimer, and lymphocyte counts. This underscores the direct relationship between tissue damage and cfDNA, making it a promising acute biomarker for assessing tissue turnover at the time of sampling.
The levels of cfDNA derived from innate immune cells, lung epithelium, and vascular endothelial cells were found to predict clinical deterioration and the duration of hospitalization. This discovery aligns with existing knowledge on the pivotal role of the innate immune system in shaping the clinical course of COVID-19.
Furthermore, the study unveiled a heightened interferon response in cell-free chromatin, revealing gene expression programs in cells before their death. This insight into the immune response could have significant implications for understanding disease progression and therapeutic interventions.
One of the most intriguing findings was the sub-clinical elevation of erythroblast and vascular endothelial cell cfDNA in asymptomatic or mild COVID-19 cases. Despite no observable symptoms related to vascular or red blood cell damage, the liquid biopsy showed evidence of increased vascular turnover and erythropoiesis, the process of red blood cell production. This suggests that SARS-CoV-2 may disrupt erythropoiesis, leading to a compensatory increase in erythroblast cfDNA.
While red blood cell counts typically remain unchanged in most COVID-19 patients, the elevated erythroblast cfDNA could indicate a higher rate of turnover, possibly resulting from a shortened lifespan of red blood cells due to viral infection or immune-mediated damage.
The implications of these findings are significant. Epigenetic liquid biopsies offer a non-invasive means of monitoring COVID-19 patients and could potentially predict the clinical course of the disease, including the development of long-covid symptoms.
Furthermore, this groundbreaking research extends beyond COVID-19 and opens new avenues for understanding complex pathologies involving tissue turnover, shedding new light on the utility of liquid biopsies in characterizing various diseases.
In conclusion, this study by the Hebrew University of Jerusalem provides a deeper understanding of the tissue dynamics in COVID-19 patients, offering critical insights into the sub-clinical vascular damage and red blood cell turnover that can occur even in asymptomatic cases. As the world continues to battle the pandemic, epigenetic liquid biopsies emerge as a promising tool in the fight against COVID-19 and other diseases that involve tissue damage and cellular turnover. Further investigations into long-covid patients will help determine the full potential of liquid biopsies as prognostic and diagnostic tools in understanding and managing this complex disease.
The study findings were published on a preprint server and are current being peer reviewed.
https://www.biorxiv.org/content/10.1101/2023.07.28.550957v1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
