Millions Of ‘Recovered’ Individuals Who Had Mild Or Asymptomatic SARS-CoV-2 Infection Are Unaware They Have Prolonged Immune Activation And Systemic Inflammation!
Source: Long COVID Nov 12, 2021 4 years, 3 months, 1 week, 4 days, 23 hours, 3 minutes ago
Long-COVID: A new study by researchers form McMaster University-Canada, University of British Columbia-Canada, University of Melbourne-Australia, St. Joseph’s Healthcare-Canada, Sinai Health-Canada and the University of Toronto-Canada has alarming revealed that mild and even asymptomatic SARS-CoV-2 infection can lead to sustained immune activation and prolonged systemic inflammation which can lead to a variety of medical symptoms which some are termed as
Long-COVID or PASC (Post-acute Sequelae of COVID-19).
It should be noted that prolonged systemic inflammation can also cause a variety of medical conditions and diseases to arise including cancers.
Typically, survivors of severe SARS-CoV-2 infections frequently suffer from a range of post-infection sequelae.
However not much is known as to whether survivors of mild or asymptomatic infections can expect any long-term health consequences.
The study team investigated lasting changes to soluble inflammatory factors and cellular immune phenotype and function in individuals who had recovered from mild SARS-CoV-2 infections (n = 22), compared to those that had recovered from other mild respiratory infections (n = 11).
Alarmingly the study findings showed that individuals who had experienced mild SARS-CoV-2 infections had elevated levels of C-reactive protein 1–3 months after symptom onset, and changes in phenotype and function of circulating T-cells that were not apparent in individuals 6–9 months post-symptom onset.
Markers of monocyte activation, and expression of adherence and chemokine receptors indicative of altered migratory capacity, were also higher at 1–3 months post-infection in individuals who had mild SARS-CoV-2, but these were no longer elevated by 6–9 months post-infection. Perhaps most surprisingly, significantly more T-cells could be activated by polyclonal stimulation in individuals who had recently experienced a mild SARS-CoV-2, infection compared to individuals with other recent respiratory infections. These data are indicative of prolonged immune activation and systemic inflammation that persists for at least three months after mild or asymptomatic SARS-CoV-2 infections.
The study findings were published in the peer reviewed journal: Viruses
https://www.mdpi.com/1999-4915/13/11/2239/htm
Many studies have focused on post-infection sequelae in survivors of severe SARS-CoV-2 infection that lead to coronavirus disease (COVID-19). However, not many have tried to understand the long-term health consequences of survivors of mild or asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.
This study analyzing long-term changes to inflammatory factors in survivors of mild COVID-19 investigates long-term changes to soluble inflammatory factors and cellular immune phenotype and function in 22 survivors of mild SARS-CoV-2 infection. The observations were then compared to 11 individuals who had recovered from other mild respiratory infections.
Interestingly patients who had experienced mild disease had elevated levels
of C-reactive protein 1–3 months after the onset of symptoms and showed changes in circulating T-cell phenotype and function that were absent 6–9 months after symptom onset.
The expression of adherence and chemokine receptors, which indicate altered migratory capacity, and markers of monocyte activation were also higher at 1–3 months post-infection in survivors of mild SARS-CoV-2 infection. However, these parameters were no longer elevated in these individuals at 6–9 months post-infection.
Also, alarming, T-cells activated by polyclonal stimulation were significantly higher in individuals who had recovered from mild SARS-CoV-2 infection than those who recovered from other respiratory infections. Thus, the observations of this study reveal prolonged immune activation as well as systemic inflammation lasting for at least 3 months after a mild or asymptomatic SARS-CoV-2 infection.
Most importantly, the study findings show that COVID-19 causes more significant immune dysregulation than that caused by other respiratory pathogen.
Though it is known that severe infections may lead to hospitalization and long-term health consequences, there is little awareness about the lasting respiratory, cardiovascular, and neurologic consequences after a mild SARS-CoV-2 infection. The range of long-term health issues and the immune pathology involving multiple organs after a SARS-CoV-2 infection shows that COVID-19 results in a greater degree of immune dysregulation than that observed after infection by other respiratory pathogens.
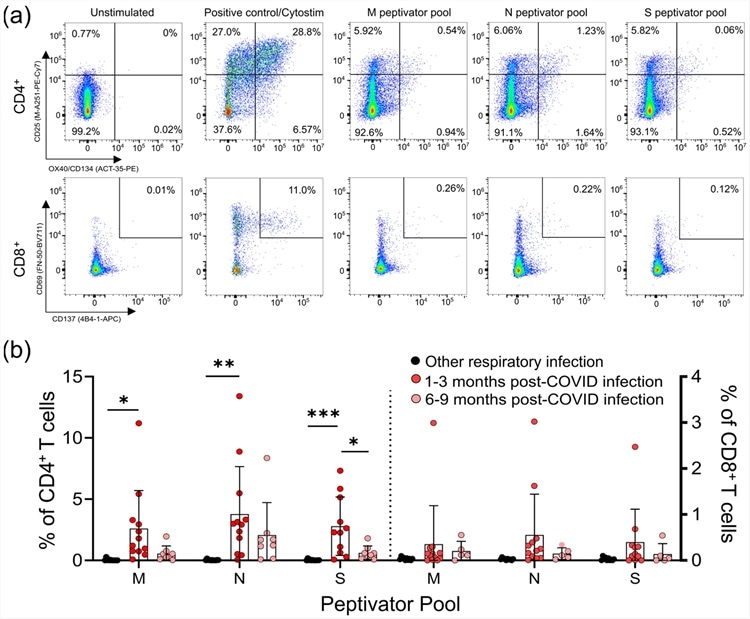 CD4+ and CD8+ T-cell responses to the M, N, and S peptide pools after mild SARS-CoV-2 infection. (a) The number of SARS-CoV-2-specific T-cells is measured as a percent of CD4+ T-cells expressing both CD25 and OX40, or CD8+ T-cells expressing both CD69 and CD137, after activation with the S, M, or N peptide pools 1–3 months and 6–9 months after infection. The polyclonal activator Cytostim is used as a positive control. (b) All COVID-19 seropositive participants had an increase in CD25+OX40+CD4+ T-cells in response to at least one of the M, N, or S antigens 1–3 months after mild COVID-19 infection, compared to seronegative individuals recovered from other mild respiratory infections. Each participant is indicated by a single data point: other respiratory infection n = 11; 1–3 months post COVID-19 infection n = 11; 6–9 months post COVID-19 infection n = 8. Multiple group comparisons were tested using Welch’s One-Way ANOVA and the Games–Howell post-hoc test; bars represent the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001.
CD4+ and CD8+ T-cell responses to the M, N, and S peptide pools after mild SARS-CoV-2 infection. (a) The number of SARS-CoV-2-specific T-cells is measured as a percent of CD4+ T-cells expressing both CD25 and OX40, or CD8+ T-cells expressing both CD69 and CD137, after activation with the S, M, or N peptide pools 1–3 months and 6–9 months after infection. The polyclonal activator Cytostim is used as a positive control. (b) All COVID-19 seropositive participants had an increase in CD25+OX40+CD4+ T-cells in response to at least one of the M, N, or S antigens 1–3 months after mild COVID-19 infection, compared to seronegative individuals recovered from other mild respiratory infections. Each participant is indicated by a single data point: other respiratory infection n = 11; 1–3 months post COVID-19 infection n = 11; 6–9 months post COVID-19 infection n = 8. Multiple group comparisons were tested using Welch’s One-Way ANOVA and the Games–Howell post-hoc test; bars represent the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001.
It should be noted that the participants in this study had only mild symptoms and did not experience “long-COVID.” Most of their symptoms resolved within 2–4 weeks. However, it is clear from the results that even participants with mild COVID-19 symptoms had elevated immune activation and inflammation for at least 1–3 months after their infection resolved. Increased systemic inflammation (e.g., CRP) and phenotypic and functional changes to monocytes and T-cells demonstrate that inflammatory responses to mild COVID-19 disease are more complicated than expected.
Shockingly, the amount of memory CD4+ and CD8+ T-cells specific for SARS-CoV-2 S, M, and N proteins observed in the study were consistent with those seen in convalescent patients who had recovered from severe COVID-19.
Hence, this implies that COVID-19 disease severity is not directly proportional to SARS-CoV-2 memory T-cell responses. SARS-CoV-2 reactive T-cells were detected in 5 survivors of mild respiratory symptoms with no positive diagnostic PCR test or seropositivity for anti-SARS-CoV-2 antibodies.
The study findings show that temporary increases in central memory CD4+ T-cells and terminally differentiated CD8+ T-cells are similar to the T-cell responses to acute viral infection.
These findings agree with previous studies showing lasting memory responses in survivors of mild SARS-CoV-2 infections. However, elevated regulatory T-cells are usually associated with minimizing pathology in the acute phase of respiratory viral infections. While severe SARS-CoV-2 infections are triggered by the development of autoantibodies that contribute to disease severity, mild SARS-CoV-2 infections are linked to autoimmune inflammatory syndromes such as arthritis or vasculitis after the primary infection is resolved.
The study findings have identified crucial differences in immune responses to SARS-CoV-2 infection and other respiratory infections. One limitation, however, is that the type(s) of non-COVID-19 respiratory infections were not determined in the study. Also, this was not a longitudinal study, so the comparison between the 1–3-month and 6–9-month post-infection groups was made using different individuals.
Corresponding author, Dr Dawn M. E. Bowdish from McMaster Immunology Research Centre at McMaster University told Thailand
Medical News, “It will be interesting to compare our data on immune responses generated by primary infection to future studies examining post-infection immune activation in vaccinated individuals with mild breakthrough infections.”
The study findings on the whole offer evidence for mild or even asymptomatic SARS-CoV-2 infections leading to sustained immune activation post symptom resolution, which does not occur in response to other mild respiratory infections. It remains to be seen if this immune activation is more prominent in patients with serious infections or long-COVID.
For more on
Long-COVID, keep on logging to Thailand Medical News.

 CD4+ and CD8+ T-cell responses to the M, N, and S peptide pools after mild SARS-CoV-2 infection. (a) The number of SARS-CoV-2-specific T-cells is measured as a percent of CD4+ T-cells expressing both CD25 and OX40, or CD8+ T-cells expressing both CD69 and CD137, after activation with the S, M, or N peptide pools 1–3 months and 6–9 months after infection. The polyclonal activator Cytostim is used as a positive control. (b) All COVID-19 seropositive participants had an increase in CD25+OX40+CD4+ T-cells in response to at least one of the M, N, or S antigens 1–3 months after mild COVID-19 infection, compared to seronegative individuals recovered from other mild respiratory infections. Each participant is indicated by a single data point: other respiratory infection n = 11; 1–3 months post COVID-19 infection n = 11; 6–9 months post COVID-19 infection n = 8. Multiple group comparisons were tested using Welch’s One-Way ANOVA and the Games–Howell post-hoc test; bars represent the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001.
CD4+ and CD8+ T-cell responses to the M, N, and S peptide pools after mild SARS-CoV-2 infection. (a) The number of SARS-CoV-2-specific T-cells is measured as a percent of CD4+ T-cells expressing both CD25 and OX40, or CD8+ T-cells expressing both CD69 and CD137, after activation with the S, M, or N peptide pools 1–3 months and 6–9 months after infection. The polyclonal activator Cytostim is used as a positive control. (b) All COVID-19 seropositive participants had an increase in CD25+OX40+CD4+ T-cells in response to at least one of the M, N, or S antigens 1–3 months after mild COVID-19 infection, compared to seronegative individuals recovered from other mild respiratory infections. Each participant is indicated by a single data point: other respiratory infection n = 11; 1–3 months post COVID-19 infection n = 11; 6–9 months post COVID-19 infection n = 8. Multiple group comparisons were tested using Welch’s One-Way ANOVA and the Games–Howell post-hoc test; bars represent the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001.