Canadian Study Find That Persistent Immune Dysfunction As Being Behind Lethality In Both COVID-19 And Non-COVID-19 Sepsis
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 27, 2023 2 years, 4 months, 1 week, 1 day, 5 hours, 59 minutes ago
COVID-19 News: The COVID-19 pandemic has reshaped the landscape of modern healthcare, revealing the immense challenges posed by a viral pathogen capable of causing severe organ failure and high mortality rates. At the forefront of this crisis, the intensive care unit (ICU) has become a battleground for the most critical cases. Here, patients afflicted with severe COVID-19 and non-COVID-19 sepsis often share a grim fate, with mortality rates reaching up to 32%. The devastating effects of both conditions are strikingly similar, characterized by life-threatening organ failure resulting from a dysregulated host response to infection, whether bacterial, viral, or fungal in origin. Sepsis, a condition estimated to claim 11 million lives annually and contribute to 1 in 5 global deaths, is an enduring healthcare challenge.
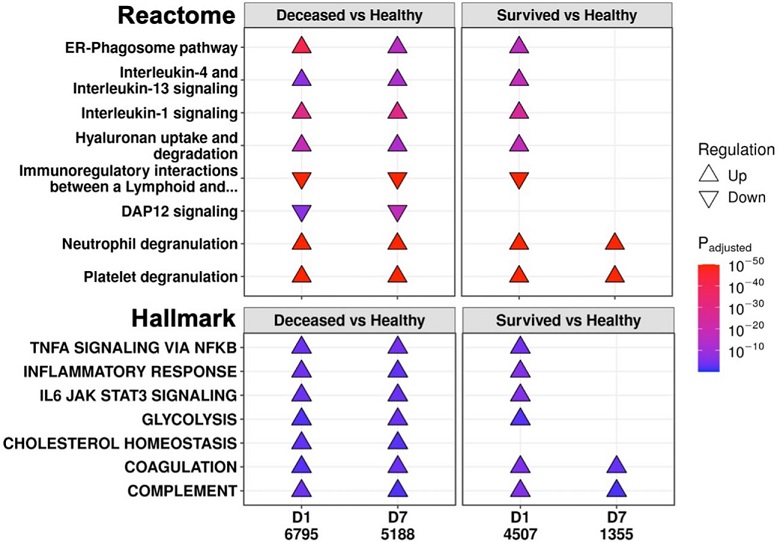 Eventually deceased patients had unresolving immune dysfunction. A subset of significantly enriched Reactome pathways (top) and Hallmark gene sets (bottom) using differentially expressed (DE) genes from comparing eventually deceased or surviving patients to healthy controls at Day 1 (D1) and Day 7 (D7). The total numbers of DE genes in each comparison are under each label. All enriched pathways and gene sets shown in Figures S6 and S7. Pathway plots were generated using pathlinkR.
Eventually deceased patients had unresolving immune dysfunction. A subset of significantly enriched Reactome pathways (top) and Hallmark gene sets (bottom) using differentially expressed (DE) genes from comparing eventually deceased or surviving patients to healthy controls at Day 1 (D1) and Day 7 (D7). The total numbers of DE genes in each comparison are under each label. All enriched pathways and gene sets shown in Figures S6 and S7. Pathway plots were generated using pathlinkR.
In light of their shared pathophysiological, immunological, and clinical features, an emerging consensus suggests that severe COVID-19 should be regarded as a form of viral-associated sepsis. Recent research conducted by the University of British Columbia, Simon Fraser University, and St. Michael's Hospital at the University of Toronto has delved into the genetic underpinnings of these two critical conditions. Their findings shed light on the commonality of immune dysfunction in severe COVID-19 and sepsis, paving the way for potential therapeutic interventions.
While numerous studies and
COVID-19 News reports have covered on the issue of COVID-19 infections leading to immune dysfunction even in the Post-COVID phases, no detailed studies to date have tracked the rapid progression of immune dysfunction in severe COVID-19 patients that are hospitalized and often ending up with fatal outcomes.
Understanding Immune Dysfunction
The lack of specific treatments for sepsis, beyond antibiotics and supportive care, has long confounded the medical community. Over three decades of clinical trials have yielded minimal success, primarily because these trials focused solely on addressing the inflammatory aspect of sepsis. However, it has become increasingly evident that sepsis involves not only inflammation but also concurrent immunosuppression, which can act as a counterbalance to limit life-threatening inflammation. This dual role of the immune system in sepsis has been recognized as "Persistent Inflammation, Immunosuppression, and Catabolism Syndrome" (PICS), a condition associated with severe sepsis characterized by recurrent infections, poor wound healing, impaired self-care, and eventual death.
The research conducted by these Canadian institutions aimed to uncover the mechanistic pathways that lead to mortality in both
severe COVID-19 and sepsis patients. Previous work by the same research group identified five distinct endotypes in early sepsis patients, two of which correlated with higher mortality rates: Neutrophilic-Suppressive (NPS) and Inflammatory (INF). A common mortality signature was also identified. Blood biomarkers, such as C-reactive protein, procalcitonin, interleukin-6, and others, have been shown to predict disease severity and mortality in both COVID-19 and sepsis patients.
Persistent Genes and Their Significance
To explore the shared mechanisms of mortality in severe COVID-19 and sepsis, the researchers collected whole blood samples from patients at two timepoints: upon ICU admission and approximately one week later. RNA-Seq analysis was performed to identify differentially expressed genes (DE genes) and enriched pathways. Notably, they discovered that non-survivors, irrespective of COVID-19 status, exhibited a significantly higher number of "persistent" genes. These persistent genes are those that remain consistently upregulated or downregulated throughout the first week of ICU admission compared to healthy controls, indicating unresolved immune dysfunction.
The researchers found that non-survivors had 3.6-fold more persistent genes than survivors, with these genes encompassing a range of immune-related pathways and genes. Importantly, these findings were replicated in publicly available datasets of COVID-19 and sepsis patients, validating the significance of persistence in immune dysfunction.
Furthermore, the study revealed that some of these persistent genes overlapped with genes identified in genome-wide association studies (GWAS) of sepsis and COVID-19 severity. Several immune-related genes, such as PCSK9, CACNA2D2, IL10RB, TYK2, and ICAM1, were among those found to be persistently dysregulated. ICAM1, in particular, was identified in GWAS studies for both sepsis and COVID-19 and was persistently upregulated only in non-survivors, suggesting a role in worse outcomes for both diseases.
Immune Pathways and Dysfunction
The functional consequences of these persistent genes in non-survivors shed light on the enduring immune dysfunction seen in these patients. Pathway enrichment analysis revealed that persistently upregulated pathways in non-survivors included those associated with interleukin (IL) and inflammatory signaling. This indicated that inflammation continued unabated in non-survivors, leading to unresolved immune dysfunction. In contrast, adaptive immune activation pathways were persistently downregulated in non-survivors, reflecting the sustained immunosuppression seen in these patients.
Interestingly, the same immune pathways found to be persistently dysregulated in severe COVID-19 and sepsis patients were also enriched in external datasets, further underscoring the shared mechanisms of mortality in these two conditions.
Adaptive immune suppression was a crucial aspect of this study's findings, with T-cell dysfunction identified as a persistent feature in non-survivors. In survivors, however, the resolution of T-cell dysfunction occurred over time, highlighting the importance of adaptive immune restoration in patient survival. This observation aligns with the concept of PICS, where immune suppression plays a pivotal role in patient outcomes.
Moreover, the study explored the influence of confounding factors such as corticosteroid use and differences in leukocyte populations. These factors did not significantly impact the study's results, reinforcing the validity of the findings.
Endotypes and Mortality Signatures
The study also assessed the utility of endotypes previously identified in emergency room sepsis patients and validated in COVID-19 patients. Two endotypes, Neutrophilic-Suppressive (NPS) and Inflammatory (INF), were associated with worse outcomes. Notably, the majority of patients who eventually died belonged to these endotypes, and many continued in these categories or transitioned to the other severe outcome endotype (INF) over time. This suggests that certain patients may be "locked-in" to more severe endotypes, emphasizing the clinical significance of these classifications.
Furthermore, the research team investigated a 38-gene mortality signature derived from early sepsis patients and found that its enrichment scores were consistently higher in non-survivors at both early and later timepoints in the ICU. The mortality signature overlapped significantly with the persistent genes in non-survivors, further emphasizing the link between immune dysfunction and mortality.
Repurposing Drugs for Therapy
Recognizing the critical role of immune dysfunction in severe COVID-19 and sepsis mortality, the study sought to identify potential therapeutic interventions. Rather than focusing on single genes, the researchers employed two systems-biology approaches to identify drugs with the potential to address the persistent genes and immune pathways seen in non-survivors.
The first approach utilized the Drug Signatures Database (DSigDB), which contains information on FDA-approved medications and their interactions with genes and proteins. This approach considered the overall systems-level effects of drugs rather than their impact on individual targets. The analysis revealed significant enrichment of anti-inflammatory drugs, including corticosteroids, which have already shown effectiveness in treating COVID-19. Surprisingly, other drugs such as antipsychotics and anti-arrhythmics were also identified, potentially through off-target inhibition of relevant pathways.
The second approach used network-based drug repurposing, considering the entire interactome of the persistent genes. This analysis identified drugs that could potentially reverse the persistent gene expression patterns observed in non-survivors. Interestingly, drugs such as tocilizumab and baricitinib, which have already shown promise in treating COVID-19 by modulating the immune response, were among the top candidates.
Additionally, a novel candidate, omeprazole, commonly used as an acid reducer, showed potential for mitigating immune dysfunction.
Conclusion
The study findings provide valuable insights into the commonality of immune dysfunction in severe COVID-19 and sepsis, underscoring the idea that severe COVID-19 can be considered a form of viral-associated sepsis. By identifying persistent genes and immune pathways associated with mortality in both conditions, this study sheds light on the shared mechanisms underlying their lethality.
The persistence of immune dysfunction, characterized by unresolved inflammation and immunosuppression, emerges as a hallmark of severe outcomes. These findings highlight the importance of timely interventions to restore immune balance in patients with severe COVID-19 and sepsis. The study also offers potential repurposed drug candidates, including corticosteroids, tocilizumab, baricitinib, and omeprazole, which warrant further investigation as treatments for immune dysfunction in these conditions.
Overall, this research provides a critical foundation for future studies aimed at improving the prognosis of patients suffering from severe COVID-19 and sepsis, offering the hope of more effective therapeutic strategies and ultimately reducing mortality rates in these devastating diseases.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1254873/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
