Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 11, 2024 1 year, 8 months, 2 weeks, 3 days, 3 hours, 23 minutes ago
COVID-19 News: In the quest to understand the complexities of COVID-19, scientists have turned their attention to the complement system, a crucial component of our innate immune defense. This system, when activated, orchestrates a powerful immune response aimed at eliminating pathogens. However, in the case of SARS-CoV-2, the virus responsible for COVID-19, this system can sometimes go into overdrive, leading to severe inflammation and tissue damage. A recent study by researchers from University of Grenoble Alpes-France and the Grenoble Alpes University Hospital-France revisits the interaction between the complement lectin pathway protease MASP-2 and the SARS-CoV-2 nucleoprotein, shedding new light on this intricate relationship that is covered in this
COVID-19 News report.
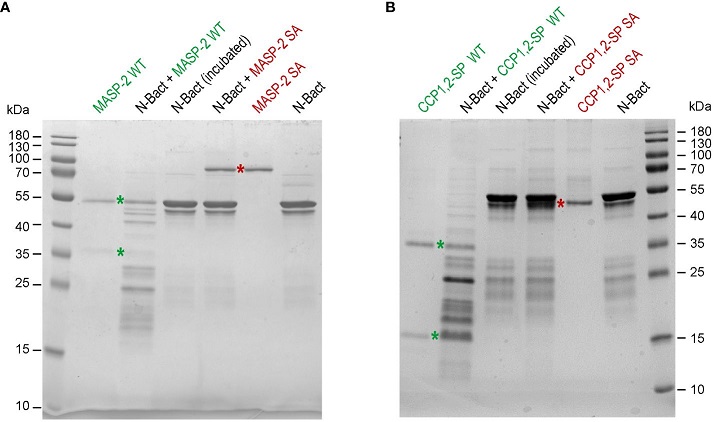 MASP-2 proteolytic activity on N-Bact protein. N-Bact was incubated with wild-type activated MASP-2 (A) or its catalytic CCP1,2-SP fragment (B), their proenzyme S618A counterparts or TBS for 90 min at 37°C. The digestion products and the control proteases and substrate were loaded on 12.5% acrylamide gels and SDS-PAGE analysis was performed under reducing conditions. The bands corresponding to the two chains of activated wild-type MASP-2 or its catalytic domain and to their proenzyme counterparts are indicated by green and red stars, respectively. The molecular masses (kDa) of the markers are indicated. Each gel shown is representative from three experiments.
The Complement System and COVID-19
MASP-2 proteolytic activity on N-Bact protein. N-Bact was incubated with wild-type activated MASP-2 (A) or its catalytic CCP1,2-SP fragment (B), their proenzyme S618A counterparts or TBS for 90 min at 37°C. The digestion products and the control proteases and substrate were loaded on 12.5% acrylamide gels and SDS-PAGE analysis was performed under reducing conditions. The bands corresponding to the two chains of activated wild-type MASP-2 or its catalytic domain and to their proenzyme counterparts are indicated by green and red stars, respectively. The molecular masses (kDa) of the markers are indicated. Each gel shown is representative from three experiments.
The Complement System and COVID-19
The complement system can be activated through three pathways: classical, lectin, and alternative. Each pathway involves different recognition proteins but converges at the cleavage of the C3 complement component. The lectin pathway, in particular, is initiated by recognizing viral surface glycoproteins by mannose-binding lectin (MBL). This recognition triggers the activation of MBL-associated serine protease (MASP-2), setting off a proteolytic cascade that contributes to the body's immune response.
In severe cases of COVID-19, strong complement activation has been observed in the serum and lungs of critically ill patients. Clinical studies have shown that blocking complement components, such as C3 or C5, can have beneficial effects. This has led to interest in the role of MASP-2 in the disease's progression.
MASP-2 and SARS-CoV-2: The Initial Hypothesis
Earlier studies suggested that the SARS-CoV-2 nucleoprotein (N protein) might interact with MASP-2, potentially causing overactivation of the lectin pathway. This hypothesis was based on the idea that such an interaction could amplify the immune response, leading to the excessive inflammation seen in severe COVID-19 cases. However, conflicting results have emerged, prompting further investigation.
Methodology: A Closer Look at Protein Interactions
To clarify the interactions between MASP-2 and the N protein, researchers conducted a series of experiments using recombinant proteins produced in both bacte
rial and mammalian cell systems. They employed surface plasmon resonance (SPR) spectroscopy to study the binding affinities and interactions between these proteins.
The researchers focused on both the full-length MASP-2 and its catalytic domain, examining them in their active and proenzyme forms. This comprehensive approach allowed them to explore the potential nuances in how these proteins might interact under different conditions.
Key Findings: Interaction and Cleavage
Contrary to previous reports, the study found no significant interaction between the SARS-CoV-2 N protein and the catalytic domain of MASP-2. However, they did observe that the N protein binds to the proenzyme form of MASP-2. This finding was surprising and suggested a more complex interaction mechanism than initially thought.
Furthermore, the researchers discovered that MASP-2 can cleave the N protein. This proteolytic activity was confirmed through incubation experiments, where the N protein was digested into smaller fragments by MASP-2. This unexpected result indicates that MASP-2 not only interacts with but also modifies the N protein, potentially altering its function.
Implications for Complement Activation
The study also explored whether the N protein influences MBL-mediated activation of the lectin pathway. They coated microplates with the viral N and S proteins and measured complement activation by C4b deposition. While the Spike (S) protein triggered robust activation, the N protein did not significantly enhance this process. This suggests that the N protein's role in complement activation is limited compared to the S protein.
However, the ability of MASP-2 to cleave the N protein adds a new layer of complexity to our understanding of these interactions. It raises questions about the potential consequences of this cleavage in the context of viral infection and the body's immune response.
Broader Context: The Complement System in COVID-19
These findings contribute to a broader understanding of how the complement system interacts with SARS-CoV-2. The complement system, while essential for defending against pathogens, can contribute to tissue damage and inflammation if not properly regulated. This study's insights into the specific interactions between MASP-2 and the N protein highlight the delicate balance within the immune system.
The results also have potential therapeutic implications. For instance, therapies targeting MASP-2, such as monoclonal antibodies, could help modulate the complement system's activity and mitigate excessive inflammation in COVID-19 patients. Understanding the precise mechanisms of these interactions is crucial for developing such targeted treatments.
Conclusion
This study revisits and refines our understanding of the interactions between the SARS-CoV-2 N protein and MASP-2. The findings suggest that while the N protein does not significantly influence lectin pathway activation, it does interact with and is cleaved by MASP-2. These insights contribute to a deeper understanding of the complement system's role in COVID-19 and pave the way for future research into targeted therapies.
By exploring these complex interactions, scientists hope to uncover new avenues for treating severe COVID-19 and other diseases where the complement system plays a critical role. As we continue to unravel the mysteries of our immune system, each discovery brings us closer to more effective and precise medical interventions.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1419165/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/post-covid-19-individuals-with-iga-nephropathy-found-to-have-overactivated-complement-system
https://www.thailandmedical.news/news/study-finds-circulating-mortalin-in-blood-and-activation-of-the-alternative-complement-pathway-as-risk-indicators-in-covid-19-infection
https://www.thailandmedical.news/news/covid-19-news-study-finds-that-variants-of-mbl2-gene-that-are-associated-with-high-mannose-binding-lectin-levels-are-protective-in-covid-19
