Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 02, 2024 1 year, 8 months, 3 weeks, 2 hours, 22 minutes ago
Cardiology Updates: Scientists from Northwestern Medicine have made a groundbreaking discovery in the field of heart disease treatment. They've found a way to regenerate damaged heart muscle cells in mice, a development that could revolutionize the treatment of congenital heart defects in children and heart attack damage in adults.
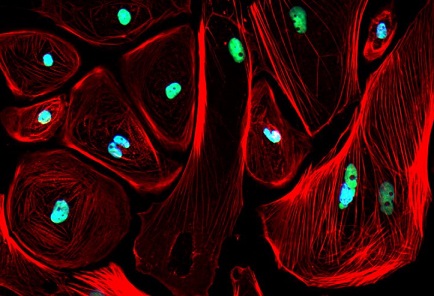 New Method Discovered for Heart Cell Regeneration
The Challenge of Heart Defects
New Method Discovered for Heart Cell Regeneration
The Challenge of Heart Defects
Hypoplastic Left Heart Syndrome (HLHS) is a rare but serious congenital heart defect that affects approximately one in 5,000 newborns. In HLHS, the left side of a baby's heart doesn't develop properly during pregnancy, leading to severe complications and often resulting in cardiac deaths within the first week of life. The Ann & Robert H. Lurie Children's Hospital of Chicago highlights the urgent need for new treatments for this condition.
The Role of Cardiomyocytes
Cardiomyocytes are the cells responsible for the contraction of the heart muscle. In newborn mammals, these cells have the remarkable ability to regenerate. However, this ability is lost as they age. Dr Paul Schumacker, the senior author of the study and a professor of Pediatrics at Northwestern, explained to journalists at
Cardiology Updates, "At the time of birth, the cardiac muscle cells still can undergo mitotic cell division. For example, if the heart of a newborn mouse is damaged when it's a day or two old, and then you wait until the mouse is an adult, if you look at the area of the heart that was damaged previously, you'd never know that there was damage there."
Reversing the Aging Process in Heart Cells
The study led by Schumacker aimed to determine whether adult mammalian cardiomyocytes could revert to their regenerative fetal state. In fetal cardiomyocytes, cellular energy is derived from glucose rather than the mitochondria. The researchers deleted a mitochondria-associated gene, UQCRFS1, in the hearts of adult mice. This genetic alteration forced the cells to return to a fetal-like state.
Remarkably, the damaged heart cells in these adult mice began to regenerate once the UQCRFS1 gene was inhibited. The cells also started to take in more glucose, mimicking the behavior of fetal heart cells. This discovery suggests that increasing glucose utilization can restore cell division and growth in adult heart cells.
Potential Treatments on the Horizon
"This is a first step to being able to address one of the most important questions in cardiology: How do we get heart cells to remember how to divide again so that we can repair hearts?" Schumacker said. Building on this discovery, Schumacker and his team are now focusing on identifying drugs that can trigger this regenerative response in heart cells without the need for genetic manipulation.
"If we could find a drug that would turn on this response in the same way the gene manipulation did, we could then withdraw the drug once the
heart cells have grown," Schumacker explained. "In the case of children with HLHS, this may allow us to restore the normal thickness to the left ventricular wall. That would be lifesaving."
Implications for Adults with Heart Damage
This approach could also be beneficial for adults who have suffered heart attacks. By enabling heart cells to regenerate, the damage caused by a heart attack could potentially be repaired, improving the patient's heart function and quality of life.
A Collaborative Effort
"This was a big project and I'm grateful to all those involved," Schumacker acknowledged. "There were 15 Northwestern faculty members who are co-authors on the paper, so it was really a team effort."
Understanding Mitochondrial Function
In addition to providing energy, mitochondria are involved in numerous other cellular functions, including signaling and regulation of cellular metabolism. In adult hearts, a transition from glycolysis (sugar-based energy production) to oxidative phosphorylation (oxygen-based energy production) occurs shortly after birth, which is associated with the loss of cardiomyocyte regenerative capacity.
The Role of UQCRFS1
The gene UQCRFS1 encodes a protein essential for mitochondrial function. Deleting this gene in adult mouse hearts led to a decrease in mitochondrial activity and an increase in glucose utilization. This metabolic shift resulted in the regeneration of cardiomyocytes and the upregulation of genes associated with heart development and cell proliferation.
Future Research Directions
Future studies will explore whether reintroducing the UQCRFS1 gene in damaged hearts can restore mitochondrial function and further improve heart regeneration. The ultimate goal is to develop treatments that can repair heart damage in both congenital conditions like HLHS and acquired conditions such as heart attacks.
Conclusion
The discovery of a method to regenerate damaged heart cells in adult mice represents a significant advancement in cardiovascular research. By understanding and manipulating the metabolic processes within heart cells, scientists hope to develop new treatments that could save lives and improve the quality of life for patients with heart disease.
The study findings were published in the peer reviewed Journal of Clinical Investigation.
https://www.jci.org/articles/view/165482
For the latest
Cardiology Updates, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/omicron-ba-2-sub-lineages-target-and-damage-cardiomyocytes-more-efficiently-than-any-other-variants
https://www.thailandmedical.news/news/research-finds-that-sars-cov-2-s-and-orf-9b-proteins-alters-metabolic-profiles-and-impairs-contractile-function-in-cardiomyocytes
