Pharmaceutical News Industry Abuzzed About Bryn Pharma Securing US$17.5 Million Funding For New Epinephrine Nasal Spray To Treat Anaphylaxis
Source: Thailand Medical News Jan 17, 2020 6 years, 1 week, 6 days, 18 hours, 15 minutes ago
The
pharmaceutical news and media segment is abuzzed about a new development that will disrupt the way that individuals with allergies or reactions having
anaphylaxis episodes are treated or able to treat themselves. A privately held Raleigh medical device company called
Bryn Pharma, is on track to soon provide people who have severe, possibly life-threatening allergies a needle-free option to treat
anaphylaxis.
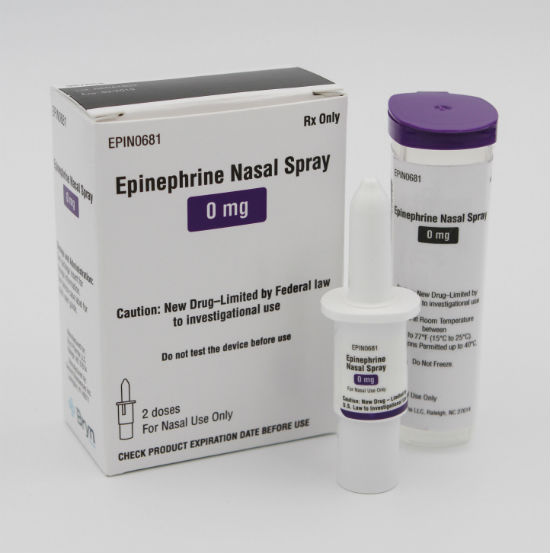
The US regulatory entity, the Food and Drug Administration granted the
Bryn Intranasal Epinephrine Spray (
BRYN-NDS1C) Fast Track designation early in 2019. More recently, the company acquired $17.5 million in new financing. Current and new investors contributed $15 million while an unnamed “corporate collaborator” invested $2.5 million.
CEO David Dworaczyk, Ph.D., told the
Thailand Medical News, “We’re excited, motivated and passionate about what we’re doing. The new financing will help get
BRYN-NDS1C into the hands of people who need.”
Roughly about 49 million Americans and more than 230 million people globally are at risk for
anaphylaxis.
Anaphylaxis is a severe overreaction of the body’s immune system to a foreign substance to which the body has become sensitized. It’s often caused by foods such as peanuts, insect stings, some medications and latex. It generally produces a sudden drop in blood pressure and narrowing of the airways, blocking breathing. Symptoms include a rapid, weak pulse; a skin rash; and nausea and vomiting.
Medical industry research indicates there are approximately 100,000 emergency department visits and an estimated 1,500 deaths attributed to
anaphylaxis each year in the United States.
For more than 30 years, the standard of care has been for patients to carry two
epinephrine auto-injectors with them at all times. The second auto-injector is recommended because up to 30 percent of people who have an anaphylactic reaction require a second dose to control symptoms.
Research and surveys have shown than less than half of patients diagnosed with
anaphylaxis carry one auto-injector. Less than 5% of those patients carry the recommended two auto-injectors because of size, cost and inconvenience. Some patients also admit to being afraid of giving themselves injections.
Dr Michelle Lobel, Bryn co-founder and chief inspiration officer, was aware of these obstacles as someone who suffers from severe nut allergies herself and as a mother with anaphylactic children. When she spoke to a doctor about treatment for her children, she was struck by the fact that it was exactly what she had been given as a child. She decided there must be a better way than
epinephrine injections.
Bryn Pharma was founded in 2016 to develop the
Bryn Intranasal Epinephrine Spray
ong> as a needle-free, portable and easy-to-use solution for treating anaphylactic reactions. The device is small enough to fit into a pants pocket or a clutch purse. One device contains two doses of epinephrine. Neither a patient nor a caregiver will need to inject anything. Instead, they’ll simply spray the life-saving medication into the patient’s nose. Should a second dose be needed, it will be available from the same device, hence no need to carry two.
Dr Dworaczyk added, “We believe our product is less intimidating and much easier to use than epinephrine auto-injectors. We see it being very disruptive and beneficial for millions of patients.”
