BREAKING NEWS! Scientists Discover That Spike Protein Of Omicron And Its Sub-Lineages Binds With Actin!
Nikhil Prasad Fact checked by:Thailand Medical News Team May 21, 2024 1 year, 8 months, 3 weeks, 5 days, 9 hours ago
COVID-19 News: Recent research conducted by Hokkaido University in Sapporo and the Japan Agency for Medical Research and Development in Tokyo has unveiled a significant discovery about the SARS-CoV-2 Omicron variant. The Omicron variant, responsible for the ongoing COVID-19 pandemic, has shown an increased affinity of its spike protein for the human angiotensin-converting enzyme type 2 (hACE2) receptor. Moreover, the study reveals that β- and γ-actin, crucial components of the cellular cytoskeleton, are specific binding partners of the Omicron spike protein's receptor binding domain (RBD). This finding covered in this
COVID-19 News report, has profound implications for our understanding of the virus's behavior and its potential long-term effects on muscle function and cell motility.
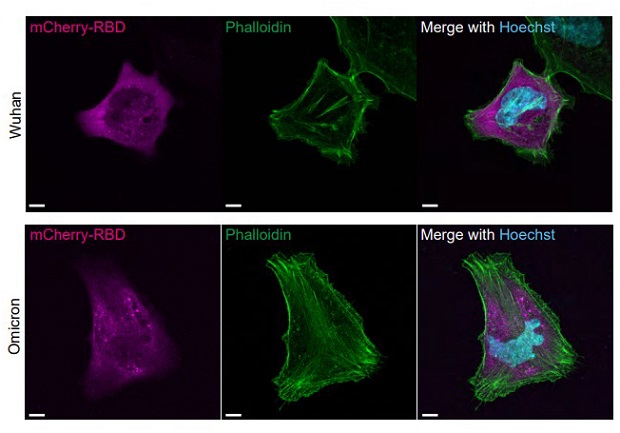 Spike Protein Of Omicron And Its Sub-Lineages Binds With Actin! Confocal fluorescent images of actin in HeLa cells expressing mCherrytagged RBD. Fluorescence images of HeLa cells expressing ER-mCherry-RBDWh1 or ER-mCherry-RBDOmic (mCherry-RBD; magenta) stained with Phalloidin-iFluor 488 (Phalloidin; green) and Hoechst 33342 for the nucleus (Hoechst; cyan). Bar = 10 µm.
The Role of Actin in Cellular Function
Spike Protein Of Omicron And Its Sub-Lineages Binds With Actin! Confocal fluorescent images of actin in HeLa cells expressing mCherrytagged RBD. Fluorescence images of HeLa cells expressing ER-mCherry-RBDWh1 or ER-mCherry-RBDOmic (mCherry-RBD; magenta) stained with Phalloidin-iFluor 488 (Phalloidin; green) and Hoechst 33342 for the nucleus (Hoechst; cyan). Bar = 10 µm.
The Role of Actin in Cellular Function
Actin is a family of globular multi-functional proteins that play a critical role in forming microfilaments in the cytoskeleton and the thin filaments in muscle fibrils. It provides mechanical support to cells, drives their movement, and participates in various biological processes, such as sensing environmental forces, internalizing membrane vesicles, and cell division. The interaction between the SARS-CoV-2 spike protein and actin suggests a novel mechanism by which the virus might affect cellular functions, particularly those related to movement and structural integrity.
The Omicron Variant and its Spike Protein
COVID-19, caused by the SARS-CoV-2 virus, has led to a global pandemic since its emergence in late 2019. The Omicron variant, first reported in South Africa in November 2021, has rapidly become a dominant strain due to its high transmissibility and ability to evade immune responses. The spike glycoprotein (S protein) on the virus's surface is crucial for recognizing human cell receptors like hACE2 and facilitating membrane fusion, allowing the virus to enter human cells. The S protein is cleaved into the S1 and S2 subunits, with the S1 subunit containing the receptor-binding domain (RBD).
Increased Affinity of Omicron RBD for hACE2
Using advanced fluorescence correlation spectroscopy (FCS) and fluorescence cross-correlation spectroscopy (FCCS), researchers demonstrated that the Omicron RBD exhibits a higher affinity for hACE2 compared to the prototype RBD. These techniques allow for precise measurement of biomolecular interactions in solution, even under conditions with low-purification levels. The study confirmed that the Omicron RBD's affinity for hACE2
is significantly higher, potentially enhancing the virus's ability to infect and transmit among humans.
Discovery of Actin as a Binding Partner
In addition to its increased affinity for hACE2, the Omicron RBD was found to interact specifically with β- and γ-actin. This discovery was made through coprecipitation experiments and confirmed by western blotting using anti-β- and anti-γ-actin antibodies. The interaction between the spike protein and actin suggests that the virus might disrupt normal cellular functions, particularly those involving the cytoskeleton and cellular movement.
Implications for Viral Infection and Cellular Function
The interaction between the Omicron RBD and actin raises several important questions about the virus's impact on cellular function. Actin is a major component of the cytoskeleton, providing structural support and driving cell movement. The binding of the spike protein to actin could potentially disrupt these processes, leading to impaired cell motility and muscle function. Moreover, the interaction between the virus and intracellular proteins might affect immune responses, cell viability, and viral infectivity.
Potential Mechanisms of Interaction
The study also explored the possible mechanisms by which the spike protein interacts with actin. Using structural predictions, researchers identified several amino acid substitutions in the Omicron RBD that are likely involved in the affinity for actin. These substitutions might enable the spike protein to bind to actin in the cytoplasm, although the exact pathway by which the protein reaches the cytoplasm remains unclear. One possibility is that the spike protein might leak into the cytoplasm during its synthesis or be retrotranslocated into the cytoplasm, similar to other viral proteins.
Summary and Future Directions
In summary, the study findings highlight a novel aspect of the SARS-CoV-2 Omicron variant's behavior. The increased affinity of the Omicron RBD for hACE2 and its specific interaction with cytoplasmic actin suggest new avenues for understanding the virus's impact on cellular function and infection dynamics. These findings underscore the importance of continued research into the molecular interactions of SARS-CoV-2 and their implications for disease progression and treatment.
Implications for Public Health
Given the potential impact on muscle function and cell motility, this discovery raises concerns about the long-term health effects of infections involving Omicron sub-lineages. Understanding these interactions will be essential for developing targeted therapies to mitigate the adverse effects of COVID-19 and improve patient outcomes.
Moving Forward
Future research should focus on elucidating the detailed mechanisms of spike protein-actin interaction and its consequences on cellular physiology. This knowledge will be invaluable in designing therapeutic strategies to counteract the virus's effects and enhance our preparedness for dealing with emerging variants.
Conclusion
The discovery that the SARS-CoV-2 Omicron spike protein binds with actin is a significant advancement in our understanding of the virus's behavior. This interaction could have far-reaching implications for the long-term effects of COVID-19, particularly concerning muscle function and cell motility. As scientists continue to unravel the complexities of the virus, these findings will be crucial in developing effective treatments and preventive measures against current and future variants.
The study findings were published on a pre-print server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.05.16.594608v1
For the latest
COVID-19-News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/university-of-florida-researchers-warns-that-covid-19-infections-can-lead-to-rhabdomyolysis-with-extremely-elevated-creatine-kinase-levels
https://www.thailandmedical.news/news/antibodies-elicited-by-inactivated-viral-vaccines-against-sars-cov-2-nsp3-found-to-also-target-host-muscle-and-neuroglial-cells
https://www.thailandmedical.news/news/covid-19-news-post-covid-respiratory-muscle-weakness-and-reduced-exercise-capacity-is-associated-with-reduced-phrenic-nerve-cmap
https://www.thailandmedical.news/news/metformin-and-leucine:-a-novel-anti-aging-tool-combating-muscle-atrophy-and-cellular-senescence
https://www.thailandmedical.news/news/long-covid-news-microvasculature-alterations,-distinct-transcriptomic-signatures-in-skeletal-muscles-and-macrophage-infiltration-found-in-long-covid
https://www.thailandmedical.news/news/alarming-study-findings-shows-that-sars-cov-2-disrupts-heart-muscle-contraction,-often-leading-to-heart-failure
https://www.thailandmedical.news/news/breaking-researchers-from-university-of-north-carolina-discover-that-the-protein-ascl1-can-help-heal-damaged-and-failing-hearts
