Nikhil Prasad Fact checked by:Thailand Medical News Team Jul 11, 2024 1 year, 7 months, 1 week, 2 days, 5 hours, 20 minutes ago
Medical News: A new study from researchers at Sun Yat-sen University Cancer Center in Guangdong, China, Brigham and Women's Hospital in Massachusetts, USA, and the Broad Institute of Harvard and MIT-USA has unveiled an innovative approach to combat viral infections. The study highlights the role of interferon-induced transmembrane proteins (IFITMs) in preventing viruses from entering host cells. This
Medical News report explores the key findings and their potential implications for future antiviral therapies.
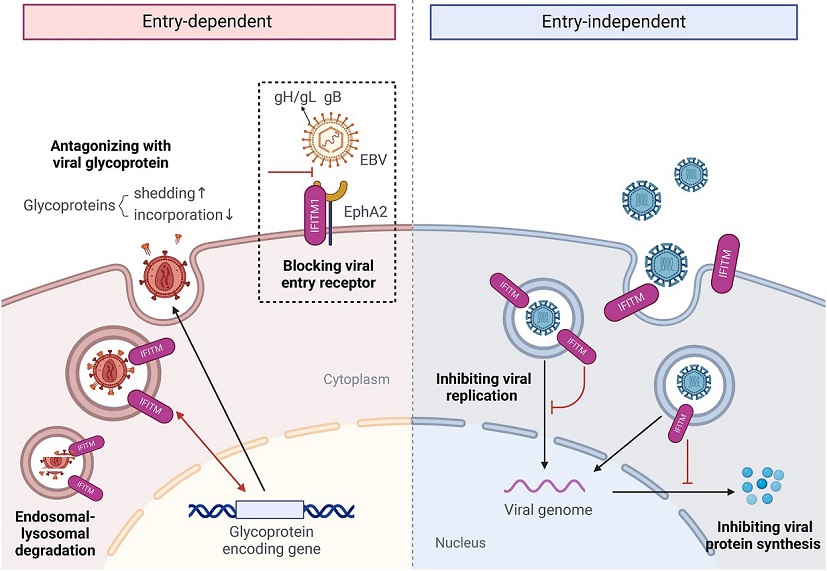 Role of IFITMs in inhibiting virus infection
Interferon-induced transmembrane proteins (IFITMs), located on the cell surface and membranes of lysosomes and endosomes, play an important role in inhibiting virus infection. Overall, the antiviral mechanisms of IFITMs can be divided into viral entry-dependent and entry-independent effects.
Image: hLife
What Are IFITM Proteins?
Role of IFITMs in inhibiting virus infection
Interferon-induced transmembrane proteins (IFITMs), located on the cell surface and membranes of lysosomes and endosomes, play an important role in inhibiting virus infection. Overall, the antiviral mechanisms of IFITMs can be divided into viral entry-dependent and entry-independent effects.
Image: hLife
What Are IFITM Proteins?
IFITM proteins are a family of proteins induced by interferons, which are crucial components of the immune response. These proteins are located on the surface of cells and on the membranes of lysosomes and endosomes inside cells. They play a significant role in inhibiting the entry of viruses into host cells. The IFITM family includes several members: IFITM1, IFITM2, IFITM3, IFITM5, and IFITM10. Each of these proteins can impact different types of viral infections through various mechanisms.
How IFITM1 Blocks Virus Entry
The researchers, led by Dr Zi-Ying Jiang, Dr Chu Xie, and Dr Pei-Huang Wu, discovered that IFITM1 can inhibit Epstein-Barr virus (EBV) infection in epithelial cells. EBV is known to enter host cells by interacting with specific receptors on the cell surface. One such receptor is the Ephrin receptor A2 (EphA2), which binds to viral glycoproteins, facilitating the virus's entry into the cell.
In this study, it was found that IFITM1 competes with EBV glycoproteins to bind to EphA2, thereby blocking the virus from entering the cells. This discovery provides a novel insight into how host cells can use their own proteins to fend off viral infections, beyond the well-known mechanisms involving antibodies and T-cells.
Key Findings of the Study
The study showed a significant negative correlation between IFITM1 expression and EBV infection in epithelial cells. When IFITM1 was knocked down, the cells became more susceptible to EBV infection. Conversely, overexpression of IFITM1 reduced the cells' vulnerability to the virus. Additionally, a soluble form of IFITM1 (sIFITM1) was also found to inhibit EBV infection in both in vitro and in vivo experiments.
The Mechanism Behind IFITM1's Protective Role
To understand how IFITM1 inhibits EBV, the researchers examined
the interactions between IFITM1 and EphA2. They discovered that IFITM1 binds to EphA2 at two critical residues, Tyr121 and Leu104, which are also the binding sites for EBV glycoproteins. By occupying these sites, IFITM1 prevents the virus from attaching to the receptor and entering the cell.
Further investigation revealed that YTHDF3, a protein involved in regulating mRNA degradation, influences the stability of IFITM1 mRNA, thereby affecting its expression levels and its ability to inhibit EBV.
Implications for Antiviral Therapies
The findings of this study suggest that IFITM1 and other members of the IFITM family could be harnessed to develop new antiviral drugs or vaccines. By boosting the expression of these proteins or administering their soluble forms, it may be possible to enhance the body's natural defense mechanisms against a wide range of viruses.
IFITM proteins have been shown to restrict infections by various viruses, including influenza A, West Nile virus, dengue virus, HIV, hepatitis C, and SARS-CoV-2. This broad-spectrum antiviral activity makes IFITM proteins a promising target for developing universal antiviral therapies.
Future Research Directions
While the study provides significant insights into the antiviral role of IFITM1, more research is needed to fully understand the mechanisms by which different IFITM proteins inhibit various viruses. It is also essential to explore the potential side effects of manipulating IFITM expression and to identify other host factors that may influence viral entry and infection.
Conclusion
This study sheds light on the crucial role of IFITM proteins in protecting host cells from viral infections by blocking key entry receptors. The findings open up new avenues for developing broad-spectrum antiviral therapies that leverage the body's natural defense mechanisms.
The study findings were published in the peer-reviewed journal: hLife.
https://www.sciencedirect.com/science/article/pii/S2949928324000609
For the latest on IFITM Proteins, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/breakthrough-german-study-discovers-that-sars-cov-2-vocs-dependent-on-ifitm2-for-replication-anti-ifitm2-antibody-inhibits-the-vocs-effectively
https://www.thailandmedical.news/news/covid-19-news-university-of-texas-study-shows-that-sars-cov-2-uses-nonstructural-protein-16-to-evade-restriction-by-ifit1-and-ifit3-
https://www.thailandmedical.news/news/research-news-yale-researchers-claim-to-uncover-new-mechanism-of-immune-cell-activation
