The structural and functional insights of the E5 helicase protein of the Mpox virus
Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 19, 2024 1 year, 6 months, 2 days, 3 hours, 32 minutes ago
Mpox News: Understanding Mpox Virus and Its Growing Public Health Concern
Mpox virus (MPXV) has emerged as a significant public health threat, particularly due to its ability to cause Mpox in humans and its rapid global spread over the past two years. The virus is a member of the orthopoxvirus genus within the Poxviridae family, which also includes the well-known Variola virus, responsible for smallpox, and the Vaccinia virus (VACV), used in smallpox vaccines.
 The structural and functional insights of the E5 helicase protein of the Mpox virus
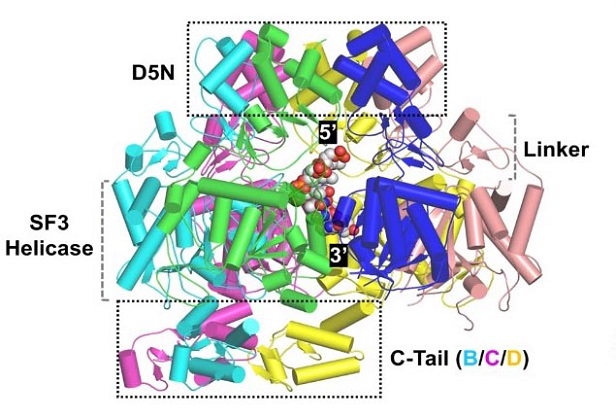
Overall folding and assembly of MPXV E5 in the complex structure.
The structural and functional insights of the E5 helicase protein of the Mpox virus
Overall folding and assembly of MPXV E5 in the complex structure.
Historically, the human-to-human transmission rate of Mpox was low, which kept the virus largely confined to the African continent until 2003. However, since May 2022, Mpox has spread across more than 150 countries, resulting in over 89,000 cases and 150 deaths as of August 9, 2023. This
Mpox News report delves into a groundbreaking study that sheds light on the structural and functional insights of the E5 helicase protein of the Mpox virus, revealing potential avenues for new therapeutic interventions.
The E5 Helicase Protein: A Key Player in Mpox Virus Replication
The study, conducted by researchers from Fudan University in Shanghai, Westlake University in Hangzhou, and other leading Chinese institutions, focuses on the E5 helicase protein of MPXV. Helicase proteins play a vital role in viral replication by unwinding the DNA double helix, allowing for the replication process to proceed. In the case of MPXV, the E5 protein is a multi-domain protein essential for both genome uncoating and DNA replication, critical steps in the virus’s life cycle.
The researchers aimed to understand the structural and functional aspects of the E5 protein to better comprehend how MPXV replicates and spreads. This article will explore the key findings of the study, which provide new insights into the virus's replication mechanisms and offer potential targets for antiviral drug development.
The Auto-Inhibited Conformation of E5: A Barrier to DNA Unwinding
One of the most intriguing discoveries of the study is that the E5 helicase protein exists in an auto-inhibited conformation, which prevents its DNA unwinding activity in vitro. This auto-inhibition means that the protein, in its natural state, cannot perform the unwinding of DNA that is necessary for the virus to replicate its genome. The researchers, however, found that by truncating the N-terminus of the E5 protein, they could recover its DNA unwinding activity. This activity was particularly evident when the protein interacted with forked DNA, a specific structure that forms during DNA replication.
This discovery is significant because it marks the first time that the DNA unwinding activity of the E5 protein has been demonstrated in vitro. The ability to unwind DNA is crucial
for the virus's replication, as it allows the viral genome to be copied and assembled into new virus particles. The findings suggest that the N-terminus of the E5 protein acts as a regulatory element that controls its activity, and by removing this element, the protein becomes active and capable of unwinding DNA.
Structural Insights from Cryo-Electron Microscopy
To uncover the detailed structure of the E5 protein, the researchers employed cryo-electron microscopy (cryo-EM), a powerful imaging technique that allows scientists to visualize proteins at near-atomic resolution. The cryo-EM studies revealed that the full-length E5 protein forms an asymmetric hexamer, a six-membered ring structure that is common among helicases. However, unlike typical helicases, the E5 protein's structure is auto-inhibited by the AEP domain, which is located at the N-terminus.
In this auto-inhibited conformation, the AEP domain blocks the entrance of DNA into the central channel of the hexamer, preventing the protein from unwinding the DNA. The truncation of the N-terminus removes this blockage, allowing the E5 protein to adopt an active conformation that can bind and unwind DNA.
The study also identified several critical residues within the E5 protein that are essential for its DNA unwinding and primer synthesis activities. These residues are located in key regions of the protein that interact with DNA and ATP (the energy molecule required for helicase activity). By mutating these residues, the researchers were able to show that they play a crucial role in the protein's function, further highlighting the importance of the E5 protein in the virus's life cycle.
Implications for Antiviral Drug Development
The discovery of the auto-inhibited state of the E5 helicase protein opens up new possibilities for antiviral drug development. One potential strategy is to develop drugs that target the auto-inhibition mechanism, preventing the E5 protein from becoming active and thereby blocking the replication of the virus. This approach could be particularly effective because the auto-inhibited conformation of the E5 protein is unique to MPXV, making it a specific target for therapeutic intervention.
Moreover, since the structure and function of helicase proteins are highly conserved across different poxviruses, including Variola and Vaccinia, these findings could lead to the development of broad-spectrum antiviral therapies. Such therapies could potentially be used to treat not only mpox but also other poxvirus infections.
Conclusion: A Step Forward in the Fight Against Mpox
In conclusion, the study provides critical insights into the structure and function of the MPXV E5 helicase protein. These findings enhance our understanding of how the Mpox virus replicates and offer promising avenues for the development of new antiviral drugs. By targeting the unique auto-inhibition mechanism of the E5 protein, it may be possible to develop treatments that can effectively halt the spread of MPXV and potentially other poxviruses.
The study findings were published in the peer-reviewed journal: Cell Discovery.
https://www.nature.com/articles/s41421-024-00680-1
For the latest
Mpox News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/a-deep-dive-into-the-immunopathogenesis-of-mpox-monkeypox-infections
https://www.thailandmedical.news/news/a-data-driven-approach-to-exploring-traditional-chinese-medicine-s-potential-in-treating-mpox
