SARS-CoV-2 RNA Replication Is Enhanced By P53-Independent G1-Cell Cycle Arrest Dependent Of The CDC25A/CDK2/Cyclin E Pathway
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 25, 2024 1 year, 11 months, 2 weeks, 6 days, 31 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has spurred an unprecedented global effort to understand its pathogenesis. Researchers from the Karolinska Institutet-Sweden, National Veterinary Institute-Sweden, Biomedrex Genetics-Sweden, Public Health Agency of Sweden, University of Barcelona-Spain, and Institució Catalana de Recerca i Estudis Avançats (ICREA)-Spain have collaborated to shed light on a crucial aspect of SARS-CoV-2 infection - the impact of cell cycle arrest on viral replication. In this comprehensive study that is covered in this
COVID-19 News report, the study team unveils a previously unrecognized mechanism: the enhancement of SARS-CoV-2 RNA replication through P53-independent G1-cell cycle arrest, dependent on the CDC25A/CDK2/Cyclin E pathway.
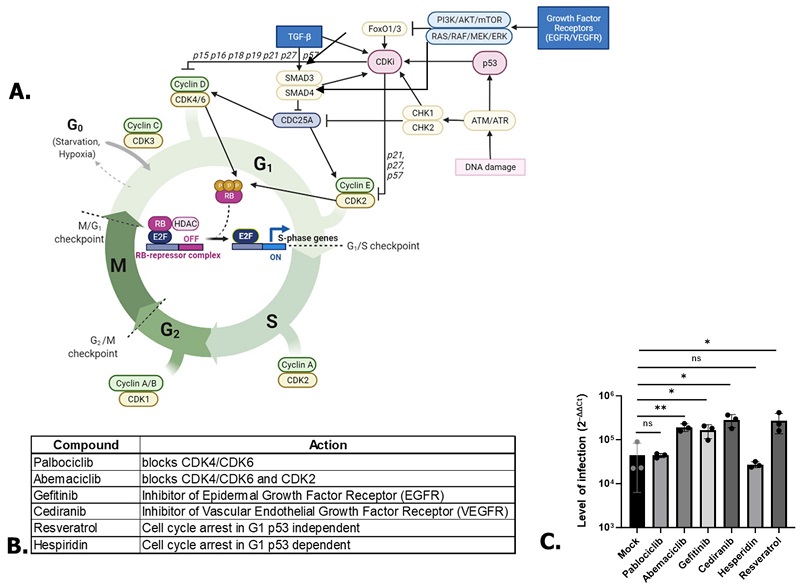 SARS-CoV-2 RNA Replication Is Enhanced By P53-Independent G1-Cell Cycle Arrest Dependent Of The CDC25A/CDK2/Cyclin E Pathway. Scheme of some proteins involved in cell cycle regulation. Created with BioRender.com. (B) Compounds used in the study associated with their targets. (C) Effect of A549 treatments on SARS-CoV-2 replication. One-way ANOVA. * p < 0.05, ** p < 0.01, ns: non-significant.
Background
SARS-CoV-2 RNA Replication Is Enhanced By P53-Independent G1-Cell Cycle Arrest Dependent Of The CDC25A/CDK2/Cyclin E Pathway. Scheme of some proteins involved in cell cycle regulation. Created with BioRender.com. (B) Compounds used in the study associated with their targets. (C) Effect of A549 treatments on SARS-CoV-2 replication. One-way ANOVA. * p < 0.05, ** p < 0.01, ns: non-significant.
Background
SARS-CoV-2, the etiological agent of COVID-19, has presented a myriad of challenges, causing flu-like symptoms that range from mild to severe, affecting not only the respiratory system but also the kidneys, gastrointestinal tract, and cardiovascular system. Despite significant progress in vaccine development and treatment strategies, the virus continues to circulate globally, emphasizing the urgency of gaining a deeper understanding of its pathogenesis for the development of novel antiviral approaches.
Eukaryotic Cell Cycle Dynamics
To comprehend the intricacies of SARS-CoV-2 replication, it is essential to delve into the eukaryotic cell cycle, a precisely regulated series of events divided into four phases: G1, S, G2, and M. The transition between these phases is tightly controlled by cell cycle checkpoints, with the G1 to S checkpoint being a critical juncture ensuring the cell's readiness for division. Cyclins and cyclin-dependent kinases (CDK) govern cell cycle progression, and their activation is a key determinant. Hypophosphorylated retinoblastoma protein (pRb) exerts inhibitory effects on the cell cycle, but upon phosphorylation by cyclin/CDK complexes, it allows progression into the S phase.
Viruses Exploiting Host Cell Functions
Viruses, including SARS-CoV and SARS-CoV-2, depend on host cell factors for replication. They often manipulate the host cell cycle to facilitate their own reproduction. Previous studies have demonstrated cell cycle arrest induced by SARS-CoV and related viruses, prompting investigations into whether SARS-CoV-2 follows a similar strategy.
Study Findings: G1-Ce
ll Cycle Arrest Boosts SARS-CoV-2 Replication
The research team conducted a series of experiments using A549 cells, an adenocarcinoma-derived human alveolar basal epithelial cell line, to decipher the relationship between cell cycle arrest and SARS-CoV-2 replication. Surprisingly, treatment with 5-(N-Ethyl-N-isopropyl)amiloride (EIPA), an inhibitor of macropinocytosis, led to a significant increase in SARS-CoV-2 infection in A549 cells. This effect was specific to SARS-CoV-2, as another RNA virus, Hazara virus (HAZV), showed deleterious effects with EIPA treatment. Importantly, EIPA-induced cell cycle arrest in G0/G1, validated through flow cytometry and pRb phosphorylation analysis, revealed that the observed increase in SARS-CoV-2 infection was independent of p53.
Confirmation through Serum Starvation and Organoid Models
To further validate the role of G1-cell cycle arrest in SARS-CoV-2 replication, serum-starved A549 cells exhibited increased infection, consistent with the observations in EIPA-treated cells. Additionally, the study extended its findings to kidney and lung organoids, demonstrating a similar enhancement of SARS-CoV-2 infection upon EIPA treatment.
Deciphering the Molecular Mechanisms: CDC25A/CDK2/Cyclin E Pathway
The study team explored the molecular pathways involved in the observed G1-cell cycle arrest and its impact on SARS-CoV-2 replication. Using various inhibitors targeting different pathways, they pinpointed the significance of the CDC25A/CDK2/Cyclin E pathway in late G1-cell cycle arrest for boosting viral replication. Notably, inhibitors of growth factor receptors and tyrosine kinases, such as Gefitinib and Cediranib, showed increased SARS-CoV-2 infection, reinforcing the pivotal role of specific pathways in viral pathogenesis.
CDC25A Degradation and CDK2/Cyclin E Inhibition
Further investigations revealed that CDC25A degradation, facilitated by EIPA treatment, was crucial for G1-cell cycle arrest. The subsequent inhibition of CDK2/cyclin E was confirmed as a key factor in enhancing SARS-CoV-2 replication. Inhibiting CDK2 with AUZ 454 or knocking down cyclin E using siRNA both resulted in increased viral infection, reinforcing the involvement of the CDC25A/CDK2/Cyclin E pathway in this process.
Implications for Therapeutic Approaches
The study's groundbreaking findings challenge the prevailing notion that P53 is a key player in SARS-CoV-2 infection. The discovery of a P53-independent G1-cell cycle arrest opens up new avenues for developing targeted antivirals. While the study acknowledges the potential of growth factor receptor inhibitors, it emphasizes the need for a nuanced understanding of the specific pathways involved to tailor antiviral strategies based on the context of infection.
Conclusion
In unraveling the intricate relationship between cell cycle dynamics and SARS-CoV-2 replication, this collaborative study provides a deeper understanding of the virus's pathogenesis. The identified P53-independent G1-cell cycle arrest via the CDC25A/CDK2/Cyclin E pathway emerges as a critical factor in enhancing SARS-CoV-2 RNA replication. These findings not only shed light on the complex interplay between the virus and host cell machinery but also pave the way for the development of targeted therapeutic approaches that could revolutionize the fight against COVID-19. As the global scientific community continues its relentless pursuit of knowledge, this study stands as a cornerstone in the ongoing battle against the SARS-CoV-2 pandemic.
The study findings were published in the peer reviewed journal: Microorganisms.
https://www.mdpi.com/2076-2607/12/3/443
For the latest
COVID-19 News, please keep on logging to Thailand Medical News.
