FDA Issues Warning on Dangers of Radiofrequency Microneedling Devices Used in Many Aesthetic or Beauty Clinics
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 04, 2025 2 months, 4 weeks, 18 hours, 35 minutes ago
Medical News: Rising Safety Concerns Over Popular Skin Rejuvenation Treatments
The U.S. Food and Drug Administration (FDA) has issued an urgent safety alert concerning radiofrequency (RF) microneedling devices, warning consumers, healthcare providers, and aesthetic clinics worldwide of serious and potentially permanent complications. RF microneedling, widely marketed as a skin “tightening” and “rejuvenation” treatment, is used to reduce wrinkles, acne scars, and signs of aging by delivering radiofrequency energy deep into the skin through an array of tiny needles.
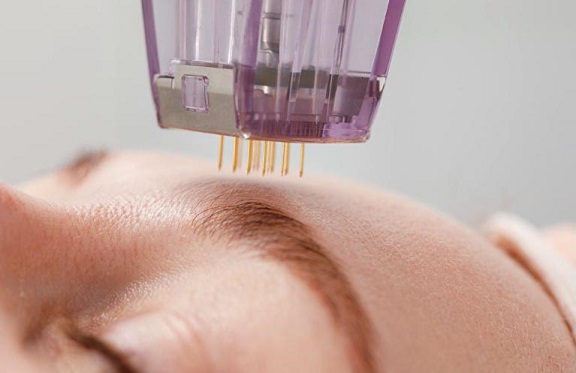 The FDA has issued a global warning after multiple reports of burns, fat loss, and scarring linked to
The FDA has issued a global warning after multiple reports of burns, fat loss, and scarring linked to
radiofrequency microneedling treatments.
According to this
Medical News report, the U.S.FDA has received numerous reports of severe adverse events linked to these procedures, including burns, scarring, fat loss, nerve damage, and permanent facial disfigurement. Some patients have required additional surgeries or medical interventions to repair the damage caused by improper use of these devices, which are often available in aesthetic clinics around the world — including Thailand.
U.S. FDA Emphasizes Medical Oversight and Prohibits Home Use
The FDA classified RF microneedling systems as Class II medical devices, meaning they require specific regulatory clearance and are meant to be used under professional medical supervision. However, the agency noted that many consumers mistakenly perceive the treatment as a routine beauty procedure rather than a medical intervention.
In its statement, the FDA stressed that RF microneedling should never be performed at home or by unlicensed operators. Only trained and certified professionals with experience handling these devices should conduct the treatment to minimize risks of burns or tissue damage. The agency is currently collaborating with device manufacturers to enhance safety guidelines and identify modifications that could reduce potential complications.
Documented Adverse Effects and Global Implications
The FDA alert was issued following a growing number of adverse event reports. Documented complications include:
• Severe thermal burns from excessive energy delivery.
• Permanent scarring and uneven skin texture.
• Facial fat atrophy leading to hollow or sunken appearance.
• Nerve injury resulting in loss of sensation or chronic pain.
• Irreversible disfigurement requiring reconstructive surgery.
The agency also cautioned that these outcomes may appear weeks after treatment, complicating diagnosis and repair. Clinics have been reminded that RF microneedling devices must be handled with caution and that patients should be fully informed of potential risks before consenting to the procedure.
FDA Urges Both Patients and Providers to Report Complications
The FDA recom
mends that patients consult licensed dermatologists or plastic surgeons before undergoing any microneedling or energy-based aesthetic procedure. It also urges immediate medical attention if redness, blistering, swelling, or unusual pain occurs after treatment. Healthcare professionals are advised to discuss all risks and alternative options with patients and to report any complications through the FDA’s MedWatch Voluntary Reporting Program to support ongoing monitoring and safety evaluation.
Growing Scrutiny of Energy-Based Aesthetic Devices
This latest warning reflects increasing regulatory scrutiny over energy-based aesthetic technologies that have gained massive popularity across Asia, the United States, and Europe. As the global cosmetic market continues to blur the lines between beauty treatments and medical interventions, authorities are emphasizing stronger oversight, stricter licensing, and better patient education to prevent harm.
The U.S. FDA’s move serves as a reminder that aesthetic enhancement procedures — no matter how minimally invasive they seem — carry real medical risks when improperly performed or marketed without adequate safety assurances. Consumers are urged to prioritize licensed medical supervision and informed consent before pursuing such treatments.
The U.S. FDA alert highlight the importance of regulatory awareness, patient safety, and transparency in aesthetic medicine. RF microneedling, while promising for skin rejuvenation, should only be undertaken in controlled clinical settings under expert care to prevent irreversible damage and protect public health.
Reference:
https://www.fda.gov/medical-devices/safety-communications/potential-risks-certain-uses-radiofrequency-rf-microneedling-fda-safety-communication
For the latest on dangers of various Aesthetic procedures, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/articles/aesthetics
