Source: Thailand Medical News Jul 04, 2019 6 years, 6 months, 4 weeks, 2 days, 9 hours, 55 minutes ago
BraveHeart Wireless Inc., a leading innovator in clinical-quality biometric wearables, has received clearance from the US FDA for the BraveHeart Life Sensor Cardiac Monitoring system.
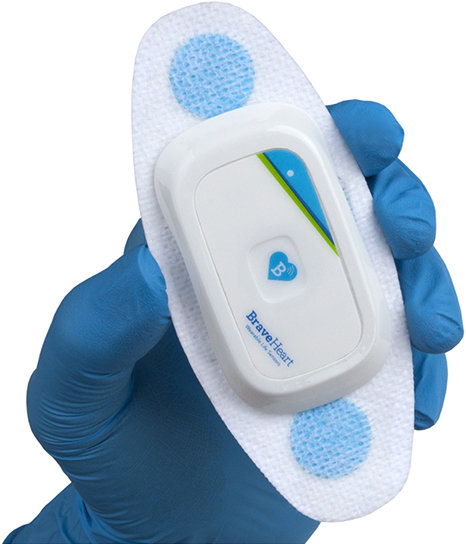
The Life Sensor Cardiac Monitoring system is intended for use by doctors and cardiologists within a health care setting. The system captures heart rate and EKG data using a Life Sensor electrode affixed to a patient’s skin and continuously transmits the data from the electrode to an application on an iOS device, where the data can be reviewed at any time by health care professionals.
Prior to the Life Sensor, patients with the need for truly wireless heart-monitoring had limited options. Now doctors can use the Life Sensor to quickly and wirelessly receive relevant, clinical-quality and actionable heart rate and EKG data,even as a patient is waiting to be admitted to a hospital..The Life Sensor has the potential to significantly and positively impact patient care. The FDA ensured the Life Sensor cardiac monitoring system met the FDA’s regulatory requirements for safety and effectiveness.
The Life Sensor Cardiac Monitoring system introduces a proprietary and innovative set of features to the cardiac monitoring segment, including these key features:
• Everyday-wear form factor. The Life Sensor Cardiac Monitoring system is the smallest, most comfortable, clinical-quality sensor patch on the market.
• Patent-pending technology. The Life Sensor Cardiac Monitoring system employs both passive and active sensors. Initial patents have been filed on BraveHeart Wireless’s critical method of multiplexing multiple sensors on a single patch.
The BraveHeart Life Sensor Internet of Things open platform is used to power the FDA-cleared Life Sensor Cardiac Monitoring system. The platform’s additional features include:
• OEM / partner channel model. The seamless integration potential of the Life Sensor Cardiac Monitoring system is designed to accelerate its penetration into multiple medical segments, from the individual researcher to large medical device entities.
• Widest array of sensors on a single patch. Because of the number and type of sensors that are built into the Life Sensor system, health care partners can rely on the Life Sensor to cover patient needs in multiple health care sectors.
• Open-platform. The Life Sensor Cardiac Monitoring system harnesses a modern, cloud-based RESTful API and Modular hardware platform. The Life Sensor Cardiac Monitoring system is designed to transmit continuous data to enable telemedicine and other modalities.
In the United States alone, 28.2 million adults are currently diagnosed with heart disease and this number continues to increase annualy. Each year, about 735,000 Americans suffer a heart attack, and heart disease has now become the leading cause of death for men and women in the United States, according to the Centers for Disease Control.
Survival rates are are greater when emergency treatment begins rapidly. The Life Sensor Cardiac Monitoring system is designed to provide fast application and access to a patient’s heart rate and EKG data.
The Life Sensor Cardiac Monitoring system is comprised of several components, including a single-use, self-adhesive
patch that can be attached to a patient’s skin directly above the heart. This patch is equipped with a sensor that can capture a patient’s heart rate and EKG data and wirelessly transmit this data via BLE (Bluetooth Low Energy) in real-time to an application on an iOS device, where the data can be reviewed by health care professionals.
For more details about Braveheartt Wireless and also the Life Sensor Cardiac Monitoring System, please visit :
www.BraveHeart.life
