Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 12, 2024 1 year, 7 months, 2 weeks, 4 days, 10 hours, 6 minutes ago
COVID-19 News: The fight against SARS-CoV-2, the virus responsible for the COVID-19 pandemic, continues as scientists uncover the intricate details of its structure and function. A recent study by researchers from the University of Chicago-USA, Purdue University-USA and Guangxi University-China that is covered in this
COVID-19 News report, has shed light on the critical role of the SARS-CoV-2 nucleoprotein (N protein) in virus assembly and its interaction with anionic lipid membranes, which are vital for the virus's lifecycle.
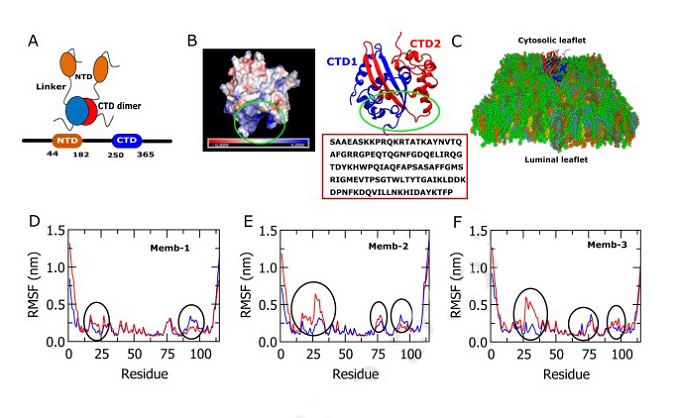 Structure of C-terminal domain (CTD) of SARS-CoV-2 N protein. Root means square fluctuations (RMSF) and electrostatic surface plots of N-CTDs. A, Schematic diagram of N protein. NTD and CTD domains are connected via a linker region where the CTD domain forms a dimer. B, The crystal structure of N protein was taken from PDB ID7DE1. The electrostatic surface plot of N-CTDs shows the membrane binding surface has a high number of positively charged residues. Red box shows the amino-acid sequence of N-CTD domain. C, N-CTD dimers bound with the lipid membrane. D, E, and F, plots show RMSF of CTD1 and CTD2 domains. The black circles point out the regions where one monomer shows higher fluctuation value compared to the other monomer, referring one CTD shows significant interactions with the lipid membrane and the other is not.
The Importance of Nucleoprotein in SARS-CoV-2
Structure of C-terminal domain (CTD) of SARS-CoV-2 N protein. Root means square fluctuations (RMSF) and electrostatic surface plots of N-CTDs. A, Schematic diagram of N protein. NTD and CTD domains are connected via a linker region where the CTD domain forms a dimer. B, The crystal structure of N protein was taken from PDB ID7DE1. The electrostatic surface plot of N-CTDs shows the membrane binding surface has a high number of positively charged residues. Red box shows the amino-acid sequence of N-CTD domain. C, N-CTD dimers bound with the lipid membrane. D, E, and F, plots show RMSF of CTD1 and CTD2 domains. The black circles point out the regions where one monomer shows higher fluctuation value compared to the other monomer, referring one CTD shows significant interactions with the lipid membrane and the other is not.
The Importance of Nucleoprotein in SARS-CoV-2
SARS-CoV-2 is a lipid-enveloped virus with a single-stranded RNA genome of approximately 30 kilobases. It encodes four major structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). Among these, the N protein is particularly abundant and essential for several functions, including RNA replication and transcription. This protein's interaction with viral RNA forms a ribonucleoprotein complex that is crucial for virus assembly.
Nucleoprotein and Lipid Membranes
The N protein's ability to bind to anionic lipid membranes is a significant finding in understanding how SARS-CoV-2 assembles and buds off from host cells. Lipid membranes are composed of various lipids, including phosphoinositides and phosphatidylserine (PS), which play essential roles in cellular functions and virus-host interactions.
Study Findings: Lipid Binding Assays
The research team employed lipid binding assays to investigate the interaction between the N protein and anionic lipids. They discovered that the N protein strongly associates with several anionic lipids, including phosphoinositides and PS. These interactions were primarily driven by electrostatic forces, as increasing the concentration of sodium chloride (NaCl) reduced the binding affinity.
In more detailed assays, such as the large unilamellar vesicle (LUV) binding assay, the N protein demonstrated a strong affinity for anionic lipid-containing vesicles compared to zwitterionic lipids. This
binding was significantly reduced in the presence of higher NaCl concentrations, further confirming the electrostatic nature of these interactions.
Nucleoprotein's C-terminal Domain: A Key Player
The study also focused on identifying which part of the N protein was responsible for lipid binding. Through truncation experiments, it was revealed that the C-terminal domain (CTD) of the N protein played a critical role in associating with anionic lipids. Constructs containing the CTD exhibited significant lipid binding, whereas those lacking it did not.
Oligomerization and Lipid Binding
Another crucial aspect of the study was to determine whether the N protein could bind to anionic lipids in its oligomeric form. The researchers used cross-linking agents to induce oligomerization and found that these oligomeric forms retained the ability to associate with anionic lipids. This suggests that the N protein can interact with lipid membranes both as monomers and oligomers, which might be important for its function in viral assembly.
Computational Insights
To complement the experimental findings, the team performed molecular dynamics simulations to understand the interactions at a molecular level. The simulations confirmed that the N protein's CTD had a positively charged surface, facilitating its interaction with negatively charged lipid head groups. Specific residues within the CTD were identified as crucial for these interactions, showing higher occupancy and longer residence times when bound to anionic lipids.
Implications for SARS-CoV-2 Lifecycle
These findings have significant implications for understanding the SARS-CoV-2 lifecycle. The interaction of the N protein with anionic lipid membranes suggests a mechanism for its recruitment to assembly sites within host cells. This lipid-dependent recruitment could be a target for therapeutic interventions, potentially disrupting virus assembly and reducing viral spread.
Future Directions
The study opens up several avenues for future research. Understanding the exact molecular mechanisms of N protein-lipid interactions can provide deeper insights into the viral assembly process. Additionally, exploring how these interactions can be disrupted by small molecules or other therapeutic agents could lead to novel treatments for COVID-19 and other coronavirus-related diseases.
Conclusion
The study findings highlight the crucial role of the SARS-CoV-2 N protein in binding anionic lipid membranes, which is essential for virus assembly and budding. This interaction is mediated by the CTD of the N protein and involves electrostatic forces. Understanding these interactions at a molecular level provides valuable insights into the viral lifecycle and potential therapeutic targets to combat COVID-19.
The study findings were published in the peer reviewed Journal of Biological Chemistry.
https://www.sciencedirect.com/science/article/pii/S0021925824019574
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/masp-2-and-sars-cov-2-n-protein-s-role-in-covid-19
https://www.thailandmedical.news/news/coronavirus-nucleocapsid-protein-suppresses-innate-immune-response-through-disruption-of-nucleocytoplasmic-trafficking
https://www.thailandmedical.news/news/covid-19-news-study-finds-that-sars-cov-2-nucleocapsid-protein-accumulates-in-renal-tubular-epithelium-of-post-covid-19-patients,-possibility-causing-
