Shock as Popular Blood Pressure Drug by Teva Pharmaceuticals Found Contaminated with Cancer Causing Chemicals
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 01, 2025 2 months, 4 weeks, 2 days, 1 hour, 5 minutes ago
Medical News: Massive Recall Sends Panic Among Hypertension Patients
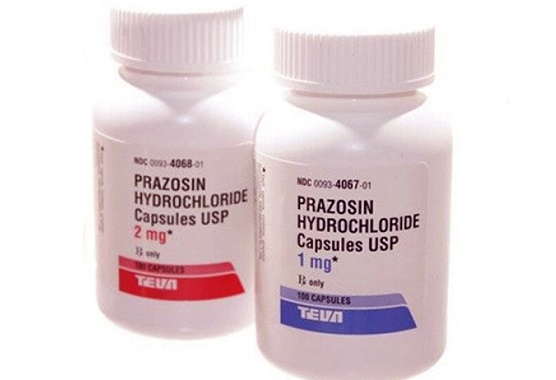
A shocking recall has been announced after the discovery that one of the most widely used blood pressure medications may be tainted with cancer-causing chemicals. Teva Pharmaceuticals USA, a major drug manufacturer based in Parsippany, New Jersey, has voluntarily recalled more than 580,000 bottles of its Prazosin Hydrochloride capsules following tests by the U.S. Food and Drug Administration (FDA) that found dangerously high levels of a toxic compound known as N-nitroso Prazosin impurity C, a type of nitrosamine associated with cancer.
S
hock as Popular Blood Pressure Drug by Teva Pharmaceuticals Found Contaminated with Cancer Causing Chemicals
The recall, issued on October 7, affects 1 mg, 2 mg, and 5 mg capsule strengths distributed nationwide. Although Teva insists that it has not yet received any reports of adverse effects, health authorities warn that the presence of these impurities poses potential cancer risks. According to this
Medical News report, the FDA has categorized the recall as a Class II risk, meaning the medication may cause temporary or reversible health effects but is unlikely to result in life-threatening harm.
What Is Prazosin and Why the Recall Matters
Prazosin, often sold under the brand name Minipress, is an alpha-blocker commonly prescribed to lower blood pressure by relaxing blood vessels, improving circulation, and reducing strain on the heart. It is also used off-label to help treat post-traumatic stress disorder (PTSD) symptoms such as nightmares and anxiety-related sleep problems. However, the recent discovery of contamination has raised alarm among doctors and patients alike.
The recall involves approximately 181,659 bottles of 1 mg capsules, 291,512 bottles of 2 mg capsules, and 107,673 bottles of 5 mg capsules—totaling over half a million bottles that may be contaminated with carcinogenic impurities. The FDA explained that nitrosamine compounds can form unintentionally during the manufacturing or storage process when specific chemical reactions occur under certain conditions, such as prolonged exposure to heat or humidity.
Health Risks and FDA’s Assessment
Nitrosamines have been linked to several types of cancer, including liver, stomach, and esophageal cancers. The FDA emphasized that while the overall health risk for short-term exposure is moderate, long-term ingestion of contaminated medication could increase cancer risk. Teva’s internal Health Hazard Assessment, reviewed by the California Board of Pharmacy, classified the patient risk as medium and confirmed that the issue only affects specific production lots.
The company has already sent recall letters to pharmacies and healthcare providers across the United States with instructions for returning affected products. Patients who are currently taking Prazosin are advised not to stop the medication abruptly but to immediately contact their doctor or pharmacist to obtain a safe alternative.
/>
Teva’s Statement and Growing Industry Concern
A spokesperson for Teva stressed that “patient safety and product quality remain top priorities” and added that the company is taking proactive steps to strengthen its manufacturing quality controls. The spokesperson also reassured consumers that “many alternative treatments for high blood pressure are readily available.”
This alarming recall is part of a wider wave of global nitrosamine-related drug recalls, which have impacted medications used to treat blood pressure, diabetes, and heartburn in recent years. The FDA and other health authorities around the world are now imposing stricter regulations to prevent these toxic chemicals from contaminating medicines in the future.
The Growing Fear Among Consumers
For millions of people relying on Prazosin to manage hypertension or PTSD symptoms, the recall has stirred fear and confusion. Many patients worry they may have unknowingly consumed tainted medication for months or even years. Medical experts emphasize that while immediate panic is unnecessary, long-term monitoring and heightened vigilance are essential to prevent similar health crises in the future.
References:
https://www.accessdata.fda.gov/scripts/ires/?Event=97755
https://www.accessdata.fda.gov/scripts/ires/?Product=216677
https://www.accessdata.fda.gov/scripts/ires/?Product=216512
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs
For the latest Drug Recalls, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/mexican-media-reports-lawsuits-against-philips-over-toxic-ventilators-linked-to-covid-19-deaths
https://www.thailandmedical.news/news/made-in-india-cough-syrups-tainted-with-toxic-deg-recalled-globally-avoid-pharma-or-supplement-products-from-india
