Immunology: University Of North Carolina Study Unveils Detailed Structure And Functioning Of DNA-Sensing Protein cGAS In Innate Immune System
Source: Immunology Sep 11, 2020 5 years, 5 months, 2 weeks, 1 day, 16 hours, 14 minutes ago
Immunology: Researchers from the University of North Carolina-Chapel Hill have in a new study for the first time, determined the high-resolution structure of a key DNA-sensing protein in the innate immune system called cGAS while it is bound to the nucleosome which is the all-important unit of DNA packaging inside a cell's nucleus.
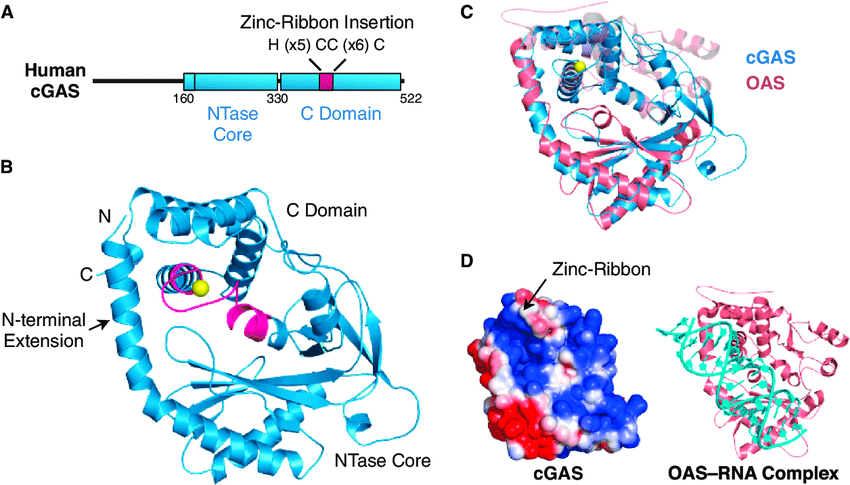
Cyclic GMP-AMP synthase or cGAS, belong to the nucleotidyltransferase family and are cytosolic DNA sensosr that activates a type-I interferon response. It is part of the cGAS-STING DNA sensing pathway. It binds to microbial DNA as well as self DNA that invades the cytoplasm, and catalyzes cGAMP synthesis which then functions as a second messenger that binds to and activates the endoplasmic reticulum protein STING to trigger type-I IFNs production.Cells lacking cGAS are more vulnerable to lethal infection by DNA or RNA viruses. In addition, cGAS has been shown to be an innate immune sensor of retroviruses including HIV. The human gene encoding cGAS is MB21D1 on chromosome 6.
The study reveals in detail how the nucleosomes inside our cells block cGAS from unintentionally triggering the body's innate immune response to our own DNA.
Cyclic GMP-AMP synthase (cGAS) recognizes cytosolic foreign or damaged DNA to activate the innate immune response to infection, inflammatory diseases, and cancer. In contrast, cGAS reactivity against self-DNA in the nucleus is suppressed by chromatin tethering.
The detailed study was led by Dr Qi Zhang, Ph.D., Associate Professor of biochemistry and biophysics at the University of North Carolina School of Medicine, and Dr Robert McGinty, MD, Ph.D., Assistant Professor of chemical biology and medicinal chemistry at the University of North Carolina Eshelman School of Pharmacy.
The study findings were published in the journal: Science
https://science.sciencemag.org/content/early/2020/09/09/science.abd0609
Co-senior author Dr Zhang told Thailand Medical News, "Detecting and responding to foreign DNA from bacterial and viral pathogens is one of the most fundamental mechanisms for host defense. A deeper understanding of functions and regulations of this important DNA sensor will have profound impacts on both basic research and translational development of cGAS-targeted therapeutics crucial to the betterment of human health."
Dr McGinty, co-senior author added, "This study was enabled by recent advances in cryo-electron microscopy technology that allows scientists, like those on our team, to observe the protein machines inside our cells with unprecedented clarity. By seeing how these proteins function normally, we can gain insights into how to manipulate their functions to treat diseases."
It has been observed in the mammalian innate immune system, the protein cyclic GMP-AMP synthase (cGAS) detects foreign or damaged "self" DNAs. Upon DNA detection, cGAS synthesizes cyclic GMP-AMP (cGAMP), the second messenger molecule that activates the cGAS-STING signaling pa
thway to fight infections, inflammatory diseases, and cancers.
As cGAS is a "universal" DNA sensor, it must be regulated to differentiate pathogenic DNA from the body's own healthy DNA to avoid any unintended immune responses. Previous research has shown that cGAS is enriched inside the nucleus where our genomic DNA is stored, but it remains a mystery as how cGAS ignores our own healthy DNA.
Utilizing the UNC School of Medicine state-of-the-art Cryo-Electron Microscopy Core Facility, which was established in 2019, the Dr Zhang and Dr McGinty labs determined a 3.3Å-resolution cryo-EM structure of cGAS in complex with the nucleosome.
The detailed structure shows that cGAS employs two conserved arginine amino acids to anchor to a negatively charged (acidic) patch on the nucleosome surface.
It was shown that these protein-protein interactions allow the nucleosome to occupy a critical DNA sensing surface on cGAS and prevent cGAS from entering its functionally active DNA-bound state. Together with mutagenesis and functional assays, this study provides a near-atomic resolution depiction of how cGAS maintains the resting, inhibited state in the nucleus.
Dr McGinty, who holds a joint faculty appointment at the UNC School of Medicine added, "These findings reshape the current paradigm of cGAS regulation and exemplify the role of the nucleosome in regulating diverse protein functions."
Dr Zhang added, "Biomedical scientists will be able to apply our research to fields such as immunology, cancer biology, and gene regulation, as well as to drug discovery for infections, inflammatory diseases, and cancers."
The team is exploring also any possible links on the cGAS-STING DNA sensing pathway and the SARS-CoV-2 mode of cellular actions on human host cells.
For the latest on
Immunology research, keep on logging to Thailand Medical News.
