Source: Thailand Medical News Nov 20, 2019 6 years, 3 months, 1 week, 1 day, 11 hours, 49 minutes ago
Rheumatoid
arthritis (RA) is a chronic inflammatory disorder that can affect more than just your joints. In some people, the condition can damage a wide variety of body systems, including the skin, eyes, lungs, heart and blood vessels. An autoimmune disorder, rheumatoid
arthritis occurs when your immune system mistakenly attacks your own body's tissues. The condition is characterized by pain and stiff joints.
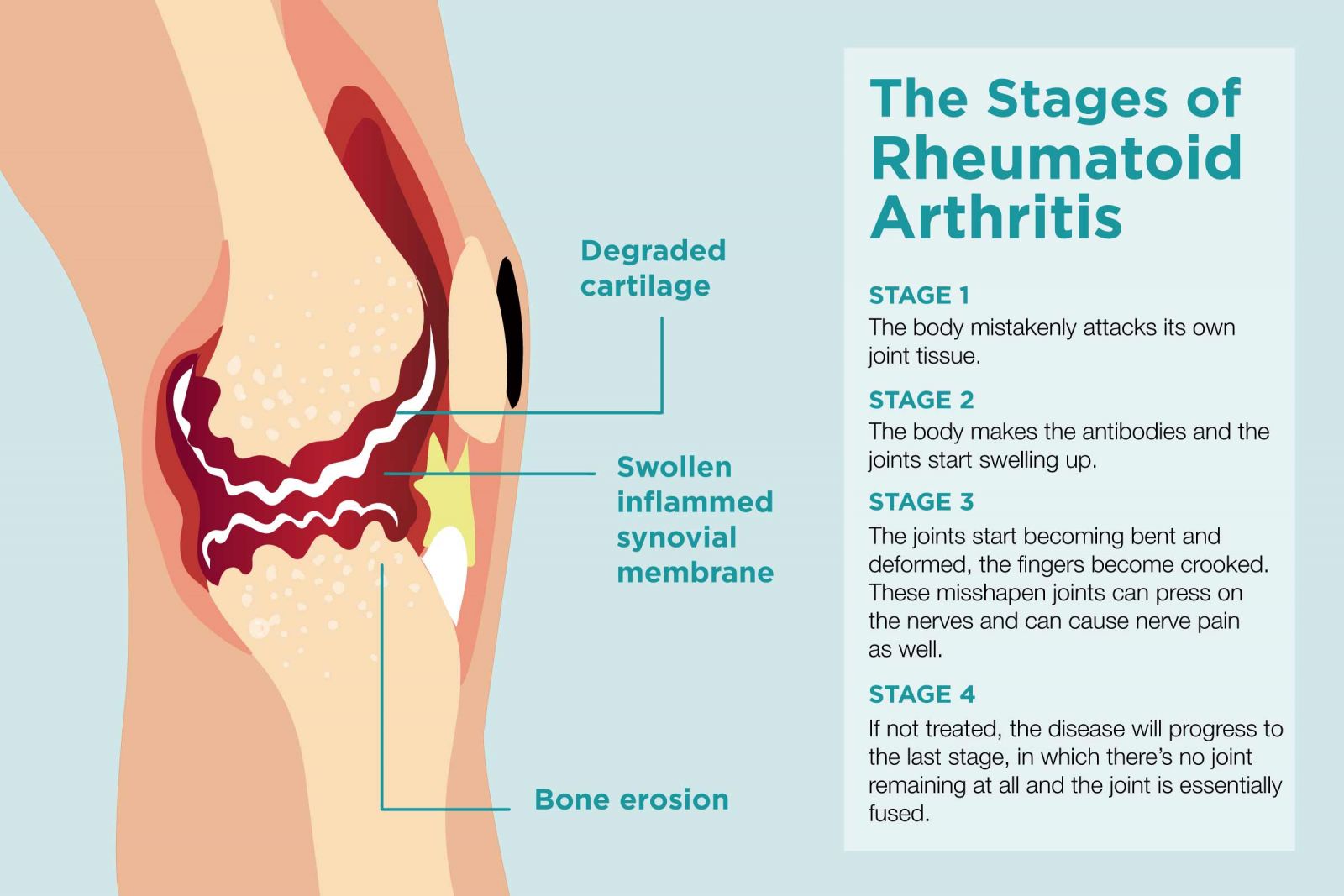
Unlike the wear-and-tear damage of osteoarthritis, rheumatoid
arthritis affects the lining of your joints, causing a painful swelling that can eventually result in bone erosion and joint deformity. Another typical feature of this disorder is the presence of autoantibodies in the serum and synovial fluid. Synovial fluid is the fluid that lubricates the synovial joints.
The detailed mechanism by which patients develop
rheumatoid arthritis is unknown; however, a combination of genetic and environmental factors is likely. Autoimmune antibody production is proposed to be the main mechanism responsible for bone and joint destruction, and the related
rheumatoid arthritis pathology. Infections, hormonal alterations, and stress are some potential triggers of
rheumatoid arthritis.
Emerging research suggests an association between
antibiotic use,
gut microbiota changes, and
rheumatoid arthritis flares.
Typically,
antibiotics are widely used for the treatment of bacterial infections associated with the respiratory system, gastrointestinal system, and urinary tract. Although antibiotics act against pathogenic bacteria, they can also modify the normal
gut microbiota.
The human
gut microbiota is a diverse system of microorganisms residing in the gastrointestinal tract of the human body.
Gut microbiota plays a vital role in maintaining the body’s digestive health.
Gut microbiota is also involved in the immune system and the synthesis of vitamin B and vitamin K.
Many new epidemiological studies have demonstrated associations between the occurrence of bacterial infections and
rheumatoid arthritis. Furthermore, microbiome alterations have been indicated as a potential mechanism for the effect of infection
in
rheumatoid arthritis pathogenesis.
Antibiotics substantially disturb the gut microbiome, with studies demonstrating significant microbial shifts in the gastrointestinal tract following their use.
The drastic alterations in the gut microbiome may last up to a year after treatment periods of only one week. As per a recent study by Nagra et al., the risk of
rheumatoid arthritis flare was significantly increased in the 1–12 months after commencing treatment on sulphonamide and trimethoprim
antibiotics.
Thailand Medical News has come across new research suggests that infections are potential risk factors for
rheumatoid arthritis pathogenesis and flares. Respiratory infections have been particularly linked with the development of
rheumatoid arthritis. Antibodies to citrullinated peptide antigens (ACPA) are one of the autoantibodies associated with
rheumatoid arthritis.
ACPAs have been found to be produced in response to certain bacterial components, which suggests the potential role of infections in
rheumatoid arthritis pathogenesis. As per a population-based study published in 2019, respiratory tract pathogens such as Chlamydia pneumoniae are associated with elevated circulating autoimmune antibodies.
Many study analysis found that the strongest association of infections and
rheumatoid arthritis was identified only in subjects treated with an
antibiotic and not in untreated subjects. These findings suggest that
antibiotic use may be the probable reason for the increased occurrence of
rheumatoid arthritis.
The studies conclude that Individuals exposed to one or more
antibiotic prescriptions were 60% more likely to develop
rheumatoid arthritis than their unexposed counterparts.
Though several studies have described the potential role of
antibiotic use on the microbiome, which is potentially disrupted in rheumatoid
arthritis, further research is required to explore the exact mechanism responsible for the same.
References:
-Sultan, A. A., Mallen, C., Muller, S., Hider, S., Scott, I., Helliwell, T., & Hall, L. J. (2019). Antibiotic use and the risk of rheumatoid arthritis: a population-based case-control study. BMC medicine, 17(1), 154. doi:10.1186/s12916-019-1394-6
-Nagra, N. S., Robinson, D. E., Douglas, I., Delmestri, A., Dakin, S. G., Snelling, S., … Prieto-Alhambra, D. (2019). Antibiotic treatment and flares of rheumatoid arthritis: a self-controlled case series study analysis using CPRD GOLD. Scientific reports, 9(1), 8941. doi:10.1038/s41598-019-45435-1
-Yoshii, K., Hosomi, K., Sawane, K., & Kunisawa, J. (2019). Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Frontiers in nutrition, 6, 48. doi:10.3389/fnut.2019.00048 