Study Shows SARS-CoV-2 Infections Downregulates ACE2 Expression While Upregulating Ang II Levels In Plasma, Resulting In Pulmonary Vascular Damage!
Source: SARS-CoV-2 Research - Medical News Jul 11, 2022 3 years, 7 months, 2 weeks, 2 days, 48 minutes ago
SARS-CoV-2 Research: A new study by researchers from the Southern University of Science and Technology, Shenzhen-China and the Guangzhou Medical University-China has shown that SARS-CoV-2 infections downregulates ACE2 expression while upregulating Ang II levels in plasma, resulting in pulmonary vascular damage!
To date, it has been found that the current ongoing worldwide COVID-19 crisis is posing a serious threat to human health, with patients reportedly suffering from thrombus, vascular injury and coagulation in addition to acute and diffuse lung injury and respiratory diseases.
The angiotensin converting enzyme 2 (ACE2) as the receptor for SARS-CoV-2 entry, is also an important regulator of renin-angiotensin system (RAS) homeostasis, which plays an unsettled role in the pathogenesis of COVID-19.
The
SARS-CoV-2 Research team demonstrated that SARS-CoV-2 Spike protein activated intracellular signals to degrade ACE2 mRNA. The decrease of ACE2 and higher level of angiotensin (Ang) II were verified in COVID-19 patients.
It was noted that high dose of Ang II induced pulmonary artery endothelial cell death in vitro, which was also observed in the lung of COVID-19 patient.
The study findings indicate that the downregulation of ACE2 potentially links COVID-19 to the imbalance of RAS.
The study findings were published in the peer reviewed Journal of Infection.
https://www.journalofinfection.com/article/S0163-4453(22)00404-2/fulltext
The angiotensin converting enzyme 2 (ACE2) or ACE2 is the cellular entry receptor for SARS-CoV-2 and plays a vital role in the renin-angiotensin system (RAS) homeostasis. It has been reported that SARS-CoV-2 infection might activate RAS and damage endothelial cells inducing thrombosis, oxidative stress, and cardiovascular dysfunction.
Already many studies have observed that myocardial damage was prevalent among patients with coronavirus disease 2019 (COVID-19).
https://pubmed.ncbi.nlm.nih.gov/32451271/
https://pubmed.ncbi.nlm.nih.gov/33428706/
https://pubmed.ncbi.nlm.nih.gov/32277408/
Another study revealed that serum from COVID-19 exhibited higher levels of angiotensin II (Ang II), which ACE2 cleaves to generate Ang 1 – 7, than in healthy controls. As such, it has been speculated that infection with SARS-CoV-2 might cause cardiovascular diseases (CVD), albeit the underlying mechanism remains poorly understood.
https://pubmed.ncbi.nlm.nih.gov/32048163/
The research team investigated the role of ACE2 in the cardiovascular pathology of COVID-19. ACE2 expression declines substantially after SARS-CoV-2 infection, and the team confirmed this by infecting Vero E6 cells and human ACE2 (hACE2)-expressing HeLa cells. Twenty-six SARS-CoV-2 proteins were transfected (using expression vectors) in hACE2-HeLa cells to identify proteins that reduced ACE2 levels.
&nbs
p;
All the cell lysates were examined by western blotting analysis. ACE2 expression was significantly reduced only in cells that expressed the viral spike protein, and the decrease in ACE2 levels was dose-dependent. Further, ACE2 expression was lower in bronchial epithelial cells collected from COVID-19 patients than in those from healthy controls.
Subsequent investigations in mice revealed that ACE2 expression in bronchial epithelial cells from virus-infected animals was significantly decreased.
The study team also co-transfected ACE2 and spike subunit 1 (S1) or 2 (S2) in HEK-293T cells and observed a significant reduction of ACE2 levels in S1-expressing cells while S2 did not affect ACE2 expression. hACE2-HeLa cells were infected with pseudoviruses coated with SARS-CoV-2 spike.
Interestingly, pseudoviral infection did not reduce ACE2 expression in the cells. Notably, incubating hACE2-HeLa cells with spike protein in vitro showed no significant decline in ACE2 levels.
The study team next infected hACE2-HeLa cells with live SARS-CoV-2 and observed for protein levels two hours and 24 hours after infection.
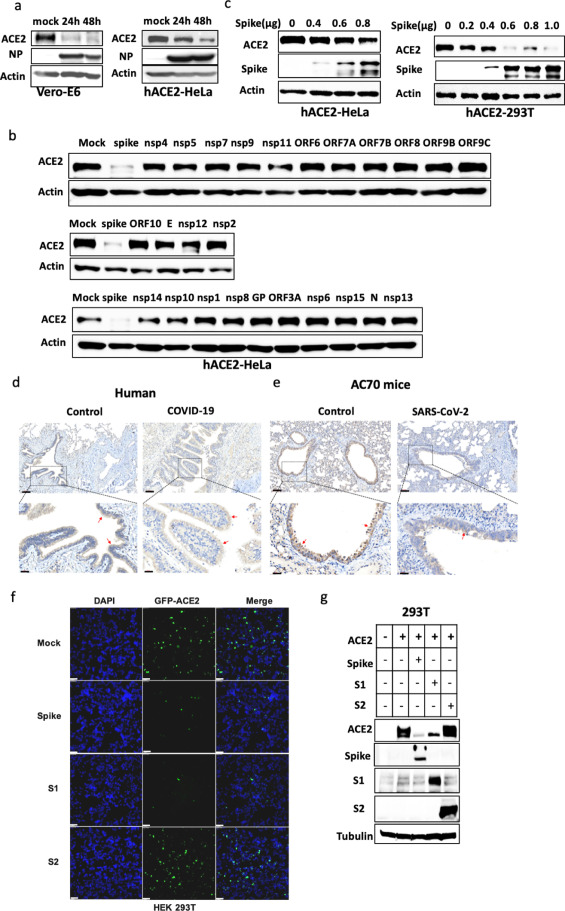 SARS-CoV-2 Spike downregulated the expression of ACE2.ACE2 was downregulated during SARS-CoV-2 infection. Vero-E6 and hACE2-HeLa cells were infected with 0.3 MOI SARS-CoV-2 for 1h, washed with PBS, and replaced with fresh media for 24h and 48h. The cell lysates were analyzed by western blotting using indicated antibodies. SARS-CoV-2 Spike can degrade ACE2. ACE2-HeLa cells were transfected with expression vectors of NSP4, NSP5, NSP7, NSP9, NSP11, ORF6, ORF7A, ORF8, ORF9B, ORF9C, ORF10, E, NSP12, NSP2, NSP14, NSP10, NSP1, NSP8, GP, ORF3A, NSP6, NSP15, N, NSP13. The cell lysates were analyzed by western blotting using ACE2 antibody and acting as the loading control. hACE2-HeLa and hACE2-293T cells were transfected with different amounts of SARS-CoV-2 Spike expression vector. The cell lysates were analyzed by western blotting using indicated antibodies. Immunohistochemistry staining of ACE2 in the lungs of healthy control or severe COVID-19 patient. Scale bars: 90 μm. The lower row shows the enlarged images of the bronchi areas above. Scale bars, 20 μm. The red arrow indicates ACE2 staining on bronchial epithelial cells. Immunohistochemistry staining of ACE2 in the lungs of control or AC70 transgenic mice infected with SARS-CoV-2. Scale bars: 90 μm. The lower row shows the enlarged images of the bronchi areas above. Scale bars, 20 μm. The red arrow indicates ACE2 staining on bronchial epithelial cells.HEK-293T cells were co-transfected with 1 μg of GFP-ACE2 expression vector and 1 μg different Spike, Spike 1 or Spike 2 plasmid for 24h. Then the cells were subjected to microscopy. The green signal shows the expression level of ACE2, and DAPI was used to stain nuclei. Scale bars: 20 μm.HEK-293T cells were co-transfected with 1 μg of ACE2 expression vector and 1 μg different Spike, Spike 1 or Spike 2 plasmid for 24h. The cell lysates were analyzed by western blotting using indicated antibodies.
SARS-CoV-2 Spike downregulated the expression of ACE2.ACE2 was downregulated during SARS-CoV-2 infection. Vero-E6 and hACE2-HeLa cells were infected with 0.3 MOI SARS-CoV-2 for 1h, washed with PBS, and replaced with fresh media for 24h and 48h. The cell lysates were analyzed by western blotting using indicated antibodies. SARS-CoV-2 Spike can degrade ACE2. ACE2-HeLa cells were transfected with expression vectors of NSP4, NSP5, NSP7, NSP9, NSP11, ORF6, ORF7A, ORF8, ORF9B, ORF9C, ORF10, E, NSP12, NSP2, NSP14, NSP10, NSP1, NSP8, GP, ORF3A, NSP6, NSP15, N, NSP13. The cell lysates were analyzed by western blotting using ACE2 antibody and acting as the loading control. hACE2-HeLa and hACE2-293T cells were transfected with different amounts of SARS-CoV-2 Spike expression vector. The cell lysates were analyzed by western blotting using indicated antibodies. Immunohistochemistry staining of ACE2 in the lungs of healthy control or severe COVID-19 patient. Scale bars: 90 μm. The lower row shows the enlarged images of the bronchi areas above. Scale bars, 20 μm. The red arrow indicates ACE2 staining on bronchial epithelial cells. Immunohistochemistry staining of ACE2 in the lungs of control or AC70 transgenic mice infected with SARS-CoV-2. Scale bars: 90 μm. The lower row shows the enlarged images of the bronchi areas above. Scale bars, 20 μm. The red arrow indicates ACE2 staining on bronchial epithelial cells.HEK-293T cells were co-transfected with 1 μg of GFP-ACE2 expression vector and 1 μg different Spike, Spike 1 or Spike 2 plasmid for 24h. Then the cells were subjected to microscopy. The green signal shows the expression level of ACE2, and DAPI was used to stain nuclei. Scale bars: 20 μm.HEK-293T cells were co-transfected with 1 μg of ACE2 expression vector and 1 μg different Spike, Spike 1 or Spike 2 plasmid for 24h. The cell lysates were analyzed by western blotting using indicated antibodies.
It was found that at 2h, ACE2 levels were unaffected but reduced significantly by 24h. This implied that internalized virion (spike protein) significantly downregulated ACE2 expression.
The research team also investigated if the spike-mediated ACE2 decline was by protein degradation and treated HEK-293T cells, after co-transfection of ACE2 and spike, with MG132 (proteasome inhibitor) and E64D (lysosome inhibitor) with dimethyl sulfoxide (DMSO) as the control. Decreased ACE2 expression was noted in cells treated with either inhibitor.
The study team also observed similar results with infection of hACE2-Hela cells.
The study findings indicated that ACE2 reduction was independent of protein degradation.
Vero E6 cells were infected with SARS-CoV-2, and total RNA was extracted for quantitative reverse-transcription polymerase chain reaction (RT-qPCR). Interestingly, ACE2 mRNA levels were significantly lower in infected cells, and the same was observed in SARS-CoV-2-infected hACE2-HeLa cells.
The study team lastly assessed if RAS was dysregulated in COVID-19 patients.
Plasma serum samples from 30 patients and 11 healthy controls were obtained. Ang II levels were significantly elevated in the sera of COVID-19 patients than in healthy controls. Treatment of human pulmonary artery endothelial cells (HPAEC) with high levels of Ang II resulted in cell death. Ang II type 1 receptor (AT1R) expression was induced upon treatment of Ang II in HPAEC.
The study team also analyzed lung tissues from one COVID-19 patient and an age-matched control for evidence of apoptosis. Endothelial cells positive for cleaved caspase 3 were detected only in the patient specimen and not the control specimen.
Higher von Willebrand factor (VWF) expression in COVID-19 specimen suggested vascular damage.
The research findings showed that SARS-CoV-2 spike protein downregulated ACE2 expression. Of note, ACE2 downregulation was not observed upon infection of HeLa cells with spike-coated pseudoviruses or when cells were treated in vitro with the spike protein. mRNA levels of ACE2 were also reduced, which might be due to modifications affecting its stability, albeit more investigation is required in the future to delineate the underlying mechanism.
Importantly, the decline in ACE2 levels was accompanied by an increase in the secretion of Ang II, affecting the endothelial cells and aggravating the risk of cardiovascular complications. Endothelial cell death in the lung tissue of a COVID-19 patient was also observed.
In conclusion, the research demonstrated that ACE2 expression decreases at protein and mRNA levels during SARS-CoV-2 infection.
It should also be noted that the damage of epithelial cells and also vascular vessels are contributing factors to heart, lung and clotting issues in Long COVID.
For the latest
SARS-CoV-2 Research, keep on logging to Thailand
Medical News.

