Influenza Vaccinations And COVID-19: Canadian Study Shows That Live Attenuated Influenza Vaccine (LAIV) Helps To Certain Degree For COVID-19
Source: Influenza Vaccinations And COVID-19 Sep 07, 2020 5 years, 4 months, 4 weeks, 1 day, 23 hours, 7 minutes ago
Influenza Vaccinations And COVID-19: A new study by Canadian researchers from Laval University and McGill University show that live attenuated influenza vaccine (LAIV) can offer a certain degree of protection against the COVID-19 disease compared to inactivated influenza vaccines (IIV) containing three or four variants.
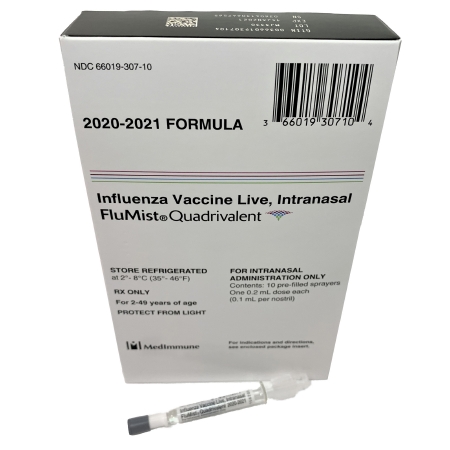
The study findings also suggest that inactivated influenza vaccines IIV should be avoided in the current COVID-19 era.
The study team came to the findings after reviewing evidence and conducting meta-analysis from 18 relevant studies on a possible interaction between influenza vaccines on non-influenza respiratory disease (NIRD).
The study findings were published on a preprint server and have yet to be peer-reviewed.
https://www.medrxiv.org/content/10.1101/2020.09.02.20186734v1
Mounting evidence shows that live attenuated vaccines offer non-specific immunity other than the specific protective effects against the target pathogens.
Such vaccines include the BCG vaccine, measles, and oral polio vaccine.
However inactivated vaccines like the diphtheria-tetanus-pertussis (DPT) vaccine are linked to a higher rate of sickness and death from other causes than the targeted diseases.
These off-target effects, whether beneficial or pathological, are often seen in children, especially for females, and might be linked to epigenetic modification of innate immunity. This is called ’trained immunity’ and results from a change in the way these cellular pathways operate as a result of the epigenetic alterations.
However certain adjuvants used in vaccines also appear to increase innate immunity and provide a broader range of protection against more than just the target microbe.
Medical experts fear that a second wave may occur in the fall, although seasonal flu rates are expected to diminish as already seen in Australia.
These experts feel that it is critical to assess the safety of the influenza vaccine in itself, and concerning a possible alteration in the risk of other respiratory infections because of influenza immunization.
The study team looked for human studies that offered any evidence that influenza vaccination and disease risk due to other respiratory pathogens, including SARS-CoV-2.
The team found, from the research findings offered by four randomized controlled trials (RCTs), that LAIV immunization did not increase the risk of respiratory infection from other pathogens. In contrast, one prospective trial in the community did demonstrate that the recipients rapidly developed non-specific immunity against viral infection hence indicating that LAIV may protect against other respiratory infections.
Alarmingly however, with IIV, six studies showed a higher risk of other respiratory infections, whether designed as RCT, cohort studies, and several observational studies/one case-control study with a test-negative design.
It should be noted that the estimated effectiveness of the influenza vaccines was independent of the control group. This could be because the flu vaccine modifies the r
isk independent of the type of pathogen, though it is more probable that no specific pathogen is inhibited.
It was observed that both innate and adaptive immune responses occur in response to the flu virus, and in animal models, this has been shown to extend to other viral infections, rapidly and durably.
Typically this involves the induction of type I interferons, TNF-α, neutrophils, macrophages, and monocytes, as well as dendritic cells and NK cells.
Interestingly when the live attenuated influenza virus is administered intranasally, it produces the immune triggering effect of wildtype influenza virus on the innate immune system.
Past research supports the safety of LAIV in the current COVID-19 scenario.
However, IIV is currently designed to stimulate adaptive immunity by increasing the production of antibodies against specific viral antigens. Animal and human studies alike show this to be less protective than LAIV-induced immunity against other influenza viruses than those included in the vaccine.
Also LAIV can enhance innate immunity via the upregulation of many interferon-related genes, but IIV elicits a gene signature that is linked to adaptive immunity, particularly related to plasma cells.
In vitro studies show that seasonal IIV can reprogram myeloid and NK cells so that their ability to counter viral infection by cytokine secretion is enhanced. However, this has not been studied in vivo by translation product analysis.
It should be noted that the mechanistic aspect of non-specific induction of antiviral protection by IIV is unknown. An increase in trained immunity that inhibits viral replication may be responsible, since by reducing the viral load, a less intense inflammatory response occurs, limiting tissue damage.
However if trained immunity does not restrict viral proliferation, on the other hand, an increase in this respect could cause viral symptoms to increase or become worse, and even trigger a cytokine storm in the more advanced phase of disease. Thus, research is required to understand if this could happen in COVID-19 following influenza immunization.
The Canadian researchers conclude that LAIV appears to be safe in the current COVID-19 scenario but not IIV.
With the small amount of data showing the possibility of harm with flu shots due to an increased risk of other respiratory viruses, it is not reasonable to stop the planned influenza immunization campaigns this year.
However a more practical solution may be to use LAIV to protect high-risk children only if possible while carrying out studies to uncover more aspects of the interactions between influenza vaccines and COVID-19 risk or disease severity.
It should also be noted that all LAIV nasal spray influenza (flu) vaccines for the 2020-2021 season are quadrivalent, meaning they will be made using four flu viruses: an influenza A(H1N1) virus, an influenza A(H3N2) virus and two influenza B viruses.
For more on
Influenza Vaccinations And COVID-19, keep on logging to Thailand Medical News.
