BREAKING! New York Study Finds That SARS-CoV-2 Virus Damages Heart Pacemaker Cells And Causes Arrhythmias!
Source: Arrhythmias and COVID-19 Apr 02, 2022 3 years, 10 months, 1 week, 3 days, 13 hours, 29 minutes ago
A new study by researchers in New York led by Weill Cornell Medicine that also included experts from Icahn School of Medicine at Mount Sinai, New York University and Columbia University Vagelos College of Physicians and Surgeons has found that the SARS-CoV-2 coronavirus is able to cause direct damage to the pacemaker cells of the heart of human host and cause arrhythmias.
To date, increasing evidence suggests that cardiac arrhythmias are frequent clinical features of COVID-19. Sinus node damage may lead to bradycardia.
It is however been challenging to explore human sinoatrial node (SAN) pathophysiology due to difficulty in isolating and culturing human SAN cells.
Fortunately, embryonic stem cells (ESCs) can be a source to derive human SAN-like pacemaker cells for disease modeling
The study team used both a hamster model and human ESC (hESC)–derived SAN-like pacemaker cells to explore the impact of SARS-CoV-2 infection on the pacemaker cells of the heart.
For the animal model involving hamsters, quantitative real-time polymerase chain reaction and immunostaining were used to detect viral RNA and protein, respectively.
The study team then created a dual knock-in SHOX2:GFP;MYH6:m Cherry hESC reporter line to establish a highly efficient strategy to derive functional human SAN-like pacemaker cells, which was further characterized by single-cell RNA sequencing.
Following exposure to SARS-CoV-2, quantitative real-time polymerase chain reaction, immunostaining, and RNA sequencing were used to confirm infection and determine the host response of hESC-SAN–like pacemaker cells.
Lastly, a high content chemical screen was performed to identify drugs that can inhibit SARS-CoV-2 infection, and block SARS-CoV-2–induced ferroptosis.
The study findings showed detection of viral RNA and spike protein in SAN cells in the hearts of infected hamsters.
The study findings show that primary pacemaker cells in the heart can be infected by SARS-CoV-2. Infection of hESC-derived functional SAN-like pacemaker cells demonstrates ferroptosis as a potential mechanism for causing cardiac arrhythmias in patients with COVID-19.
The study team also identified two drug candidates, deferoxamine and imatinib that that can protect the SAN cells from SARS-CoV-2 infection.
The study findings were published in the peer reviewed journal: Circulation Research by AHA (American Heart Association).
https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.121.320518
This preclinical study confirms that the SARS-CoV-2 virus can infect specialized pacemaker cells that maintain the heart’s rhythmic beat, setting off a self-destruction process within the cells.
The study findings offer a possible explanation for the heart arrhythmias that are commonly observed in patients with SARS-CoV-2 infection.
The study team used an animal model as well as human stem cell-derived pacemaker cells to show that SARS-CoV-2 can readily infect pacemaker cells and trigger a process called ferroptosis, in which the cells self-destruct but also produce reactive oxygen molecules that can impact nearby cells.
<
;br />
Co-senior author Dr Shuibing Chen, the Kilts Family Professor of Surgery and a professor of chemical biology in surgery and of chemical biology in biochemistry at Weill Cornell Medicine told Thailand
Medical News, “This is a surprising and apparently unique vulnerability of these cells. We looked at a variety of other human cell types that can be infected by SARS-CoV-2, including even heart muscle cells, but found signs of ferroptosis only in the pacemaker cells.”
To date, arrhythmias including too-fast (tachycardia) and too-slow (bradycardia) heart rhythms have been noted among many COVID-19 patients, and multiple studies have linked these abnormal rhythms to worse COVID-19 outcomes. How SARS-CoV-2 infection could cause such arrhythmias has been unclear, though.
The study team examined golden hamsters ie one of the only lab animals that reliably develops COVID-19-like signs from SARS-CoV-2 infection and found evidence that following nasal exposure the virus can infect the cells of the natural cardiac pacemaker unit, known as the sinoatrial node.
In order to study SARS-CoV-2’s effects on pacemaker cells in more detail and with human cells, the study team used advanced stem cell techniques to induce human embryonic stem cells to mature into cells closely resembling sinoatrial node cells.
The study team showed that these induced human pacemaker cells express the receptor ACE2 and other factors SARS-CoV-2 uses to get into cells and are readily infected by SARS-CoV-2.
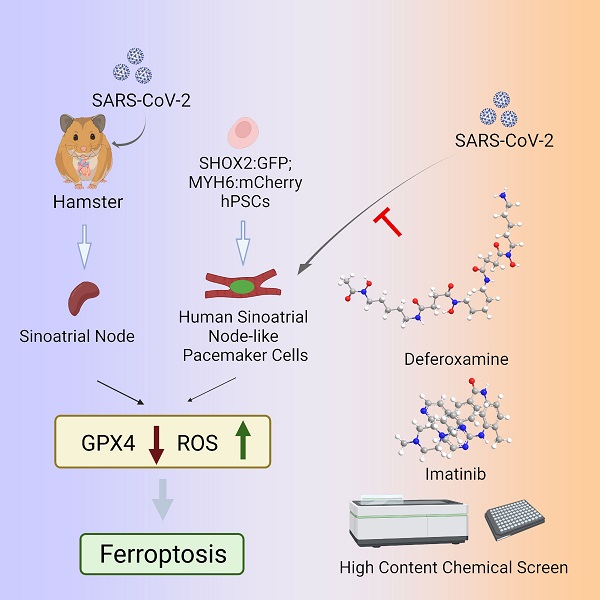 Graphical Abstract
Importantly, the study team also observed large increases in inflammatory immune gene activity in the infected cells.
However, the most shocking findings was that the pacemaker cells, in response to the stress of infection, showed clear signs of a cellular self-destruct process called ferroptosis, which involves accumulation of iron and the runaway production of cell-destroying reactive oxygen molecules.
Graphical Abstract
Importantly, the study team also observed large increases in inflammatory immune gene activity in the infected cells.
However, the most shocking findings was that the pacemaker cells, in response to the stress of infection, showed clear signs of a cellular self-destruct process called ferroptosis, which involves accumulation of iron and the runaway production of cell-destroying reactive oxygen molecules.
The study team was able to reverse these signs in the cells using compounds that are known to bind iron and inhibit ferroptosis.
Co-senior author Dr Robert Schwartz, an associate professor of medicine in the Division of Gastroenterology and Hepatology at Weill Cornell Medicine and a hepatologist at New York-Presbyterian/Weill Cornell Medical Center added, “This study finding suggests that some of the cardiac arrhythmias detected in COVID-19 patients could be caused by ferroptosis damage to the sinoatrial node.”
The study team stressed that although in principle COVID-19 patients could be treated with ferroptosis inhibitors specifically to protect sinoatrial node cells, antiviral drugs that block the effects of SARS-CoV-2 infection in all cell types would be preferable.
The study team plan to continue to use their cell and animal models to investigate sinoatrial node damage in COVID-19 and beyond.
Co-senior author Dr Todd Evans, the Peter I. Pressman M.D. Professor of Surgery and associate dean for research at Weill Cornell Medicine added, “There are other human sinoatrial arrhythmia syndromes we could model with our platform. And, although physicians currently can use an artificial electronic pacemaker to replace the function of a damaged sinoatrial node, there’s the potential here to use sinoatrial cells such as we’ve developed as an alternative, cell-based pacemaker therapy.”
The study findings also have implications for long COVID conditions and also the rising excess deaths attributed to heart failures.
Thailand Medical News would like to add that this study finds add to the list of ways that the SARS-CoV-2 virus ad its viral proteins can affect the human host heart ie from myocarditis, arrythmias and damage to other cells and tissues of the heart directly or via cytokine storm induced effects. It has also been found that the virus can also affect a variety of cellular pathways that can dysregulate a variety of genes associated with the heart.
A study currently underway has also found that the new BA.2 variant and its emerging subvariants can cause indirectly the dysregulation of a gene called MAPRE2 that plays a critical role in the functioning of the heart muscles.
For that reason, among many others that we have discovered, we at Thailand Medical News are also asking individuals out there to stop using the drug fluvoxamine to treat COVID-19 as promoted by some morons as fluvoxamine itself is able to cause arrythmias and can actually increase the risk of heart failure for people with COVID-19 especially those infected with the BA.2 variant or subvariants.
https://www.thailandmedical.news/news/most-who-have-been-exposed-to-the-proteins-of-the-sars-cov-2-virus-will-have-shortened-lifespans-stop-using-fluvoxamine-for-ba-2-infections
For more on
Arrythmias and COVID-19, keep on logging to Thailand Medical News.

