Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 10, 2024 1 year, 3 months, 2 weeks, 6 days, 13 hours, 26 minutes ago
Medical News: COVID-19 has brought various health complications since it first emerged in December 2019. While initially considered a respiratory illness, research has uncovered its impact on multiple organs, including the heart. This Medical news report delves into the possibility of COVID-19 causing a condition called "myocardial fibrosis," a thickening of the heart tissue that can result in long-term damage. A team of researchers from multiple institutions, including the Department of Urology at The First Affiliated Hospital of Zhengzhou University in China, along with experts from Shanghai Jiao Tong University, Tianjin Lung Cancer Center, and Nanjing Medical University, have contributed to this important study. The findings from their research shine light on the hidden risks of COVID-19.
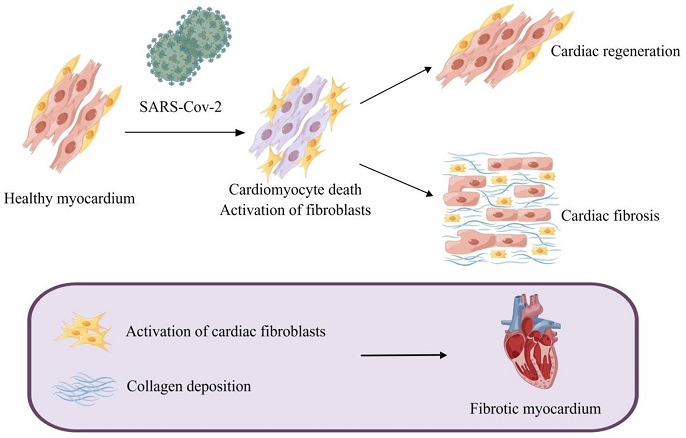 Myocardial fibrosis caused by SARS-CoV-2. SARS-CoV-2-induced cardiomyocyte death leads to activation of myofibroblasts and a reparative fibrotic response in the injured region. Activation of cardiac fibroblasts and deposition of collagen eventually lead to myocardial fibrosis.
Myocardial fibrosis caused by SARS-CoV-2. SARS-CoV-2-induced cardiomyocyte death leads to activation of myofibroblasts and a reparative fibrotic response in the injured region. Activation of cardiac fibroblasts and deposition of collagen eventually lead to myocardial fibrosis.
As early as February 2023,
Thailand Medical News had published an article warning about cardiac fibrosis in post-COVID individuals which is difficult to detect and can be asymptomatic in early stages and can cause sudden deaths.
https://www.thailandmedical.news/news/breaking-covid-19-news-wisconsin-study-shows-that-sars-cov-2-causes-cardiac-fibrosis-many-could-die-suddenly
What is Myocardial Fibrosis?
Myocardial fibrosis is a condition where the heart’s tissue becomes thickened due to an excess buildup of collagen, leading to reduced elasticity and function of the heart. Typically, this process is triggered by the body's response to injury or stress, with COVID-19 being one such trigger. As the virus attacks the body, it initiates various immune and fibrotic responses, particularly affecting cardiac fibroblasts - cells responsible for maintaining the heart’s structure. These fibroblasts become overactive in response to the viral infection, leading to the development of fibrotic tissue in the heart.
The Role of SARS-CoV-2 in Myocardial Fibrosis
The virus primarily enters the body through ACE2 receptors found in various organs, including the heart. Once inside the body, SARS-CoV-2 disrupts normal cellular functions, causing heart cells to die and promoting the activation of fibroblasts. The activation of these fibroblasts contributes to the stiffening of the heart muscles. The researchers identified several molecular pathways, including the renin-angiotensin-aldosterone system (RAAS) and transforming growth factor-beta 1 (TGF-β1), as key players in the fibrotic response. These pathways cause inflammation, cell death, and excessive tissue growth, worsening the heart’s ability to pump blood effectively.
Renin-Angiotensin-Aldosterone System (RAAS)
The RAAS is a hormone system that regulates blood pressure and fluid balance. In the case of COVID-19, this system becomes overactive, leading to the production of angiotensin II (Ang II), a protein that directly promotes fibrosis. SARS-CoV-2 exacerbates this by reducing the body's ability to produce angiotensin 1–7 and angiotensin 1–9, which usually work to counteract the fibrotic effects of Ang II. As a result, Ang II continues to activate harmful processes that cause more fibrosis in the heart.
Transforming Growth Factor-Beta 1 (TGF-β1)
TGF-β1 is another significant contributor to myocardial fibrosis. This molecule not only promotes fibrotic processes but also suppresses the immune system, allowing the virus to continue damaging the heart. In patients with severe COVID-19, researchers found elevated levels of TGF-β1, which correlated with worse outcomes. These findings highlight the importance of monitoring TGF-β1 levels in COVID-19 patients to identify those at higher risk for long-term heart complications.
MicroRNAs and Their Role in Fibrosis
MicroRNAs (miRNAs) are small molecules that regulate gene expression. In COVID-19 patients, certain miRNAs, such as miR-21 and miR-155, are significantly increased. These molecules stimulate pathways that activate fibroblasts, furthering the development of fibrotic tissue. On the other hand, miR-29, which normally helps regulate fibrosis, is reduced in COVID-19 patients. The imbalance between these miRNAs creates a favorable environment for fibrosis to develop, making them potential targets for future treatments aimed at preventing long-term heart damage from COVID-19.
Manifestations and Consequences of Myocardial Fibrosis
As myocardial fibrosis progresses, patients may experience reduced heart function, leading to symptoms such as shortness of breath, chest pain, and fatigue. Even after recovery from the acute phase of COVID-19, many patients report lingering heart-related symptoms. This is because the fibrotic process can continue long after the virus has been cleared from the body. Studies have shown that up to 60% of patients hospitalized with COVID-19 exhibit signs of myocardial injury, and a significant number develop persistent symptoms months after recovery.
Immune Cells and Inflammation
The immune response to COVID-19 also plays a critical role in the development of myocardial fibrosis. Macrophages and T-cells, key components of the immune system, infiltrate the heart during infection and release cytokines that promote fibrosis. These immune cells, which are meant to protect the body, inadvertently contribute to long-term damage by triggering fibrotic processes. Researchers found that in severe cases of COVID-19, macrophages shifted to a pro-fibrotic state, furthering the damage to the heart.
The Future of Treatment for Myocardial Fibrosis
While current treatments for COVID-19 focus primarily on controlling the virus and managing symptoms, there is growing interest in developing therapies that target myocardial fibrosis directly. Inhibitors of the RAAS, such as angiotensin-converting enzyme (ACE) inhibitors, have shown promise in reducing fibrosis by blocking the effects of Ang II. Similarly, TGF-β1 inhibitors and miRNA therapies are being explored as potential options for preventing long-term heart damage. However, more research is needed to determine the most effective strategies for treating COVID-19-associated myocardial fibrosis.
Conclusion
The findings from this study highlight the potential for COVID-19 to cause long-term heart damage through the development of myocardial fibrosis. By identifying key molecular pathways, such as RAAS and TGF-β1, the researchers provide a roadmap for future studies aimed at preventing and treating this condition. The discovery of miRNA imbalances in COVID-19 patients also opens up new avenues for research into targeted therapies that could mitigate the fibrotic process. As we move into the post-pandemic era, it will be essential to continue monitoring the long-term effects of COVID-19 on heart health and to develop interventions that address the underlying causes of myocardial fibrosis.
The study findings were published in the peer-reviewed journal: Frontiers in Microbiology.
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2024.1470953/full
For the latest COVID-19 News, keep on logging to
Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-news-german-study-find-that-30-percent-of-individuals-with-long-covid-have-non-ischemic-myocardial-fibrosis
https://www.thailandmedical.news/news/covid-19-is-not-mild-as-most-will-develop-lung-fibrosis-10-percent-of-all-lung-transplants-in-u-s-now-go-to-post-covid-patients
