Alarming New Study Data Validates That SARS-CoV-2 Spike Proteins Damages Not Just Lung Cells But Also Intestinal Cells! Many Are Dying Slowly And Silently!
Source: Medical News -SARS-CoV-2 Spike Proteins Destroys Intestinal Cells. Sep 04, 2022 3 years, 5 months, 1 week, 5 days, 3 hours, 33 minutes ago
Data from a new study by researchers from Shenzhen Baoan Second People's Hospital in China has alarmingly validated that not only does the spike proteins of the SARS-CoV-2 virus damage lung cells but they also damage and destroy the intestinal cells as well!
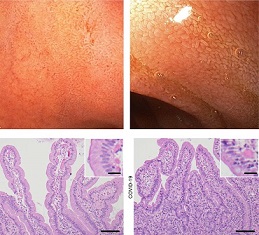
The study findings clearly showed that the SARS-CoV-2 spike protein damaged human small intestinal mucosa epithelial cells (HSIMECs), human colonic epithelial cells (HCoEpiCs), and human type II alveolar epithelial cells (hTIIAECs) which was associated with cytokine production and the induction of apoptosis mediated by the TGF-β/Smad3, but not the NF-κB, pathway.
The study data alerts medical researchers, doctors and infected individuals to numerous worrisome long-term health scenarios and alarming indicates that many could be slowly and silently dying not only due to exposure to the virus through natural infections but also possibly due to therapeutic exposures!
The study findings were published on a preprint server and is currently being peer reviewed for publication into the journal: Molecular Biology Reports By Springer.
https://www.researchsquare.com/article/rs-1999623/v1
The COVID-19 disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus), continues to ravage the world.
To date, according to official figures more than 610.2 million people have been infected with the SARS-CoV-2 virus and its various emerging variants or subvariants and more than 6.5 million people have died form COVID-19. Realistically the actual figures are believed to be as high as 5-fold! Excess death rates are also skyrocketing around the world.
Even with many prevention and control measures, morbidity and mortality have not decreased due to SARS-CoV-2-induced organ damage, which occur via unknown mechanisms.
In this new research, the study team treated primary Human small intestinal mucosa epithelial cells (HSIMECs), human colonic epithelial cells (HCoEpiCs), and human type II alveolar epithelial cells (hTIIAECs) with recombinant SARS-CoV-2 S protein for 48 h.
Cell morphology, permeability, and viability were detected. The expression of cysteinyl aspartate specific proteinase 3 (caspase 3) and B-cell lymphoma-2 (Bcl-2) was examined using Western blotting. Enzyme-linked immunosorbent assay (ELISA) was performed to detect the levels of inflammatory cytokines in the supernatant.
Apoptosis was observed using a Hoechst 33258 Staining Kit. SB431542 and BAY11-7082 were used to inhibit transforming growth factor-β (TGF-β)/suppressor of mothers against decapentaplegics (Smads) and the inhibitor of kappa B kinase (IKK)/nuclear factor-κB (NF-κB) pathways, respectively.
The study findings showed that SARS-CoV-2 spike protein produced no obvious changes in morphology, but decreased cell viability and increased permeability were observed in a concentration- and time-dependent manner compared to the control (P<0.05).
Apoptosis occurred with increased caspase 3 and decreased Bcl-2 (P<0.05). S protein stimulated a disordered secretion of cytokines, including interleukin (IL)-6, IL-10, tumour necrosis factor α (TNF-α), and IL-13 (P<0.05).
;
Suppression of TGF-β/Smad3, but not the IKK/NF-κB, pathway relieved the damage to colon cells caused by the S protein in HSIMECs and HCoEpiCs and inhibited apoptosis mediated by TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) in hTIIAECs.
Corresponding author, Dr Weizeng Shen from Shenzhen Baoan Second People's Hospital told Thailand
Medical News, “Our study findings showed and validated that SARS-CoV-2 spike protein damaged intestine and lung cells, which was associated with cytokine production and the induction of apoptosis mediated by the TGF-β/Smad3, but not the NF-κB, pathway.”
Numerous past studies have already showed that SARS-CoV-2 infections causes cytotoxic effects in several human cells, such as cardiomyocytes, immune cells, and intestinal cells. Increasing clinical and experimental data demonstrated that SARS-CoV-2 induced injury in a variety of cells and tissues, including myocardial cells, endothelial cells, pulmonary microvascular transendothelial cells, and alveolar type 2 cells.
https://pubmed.ncbi.nlm.nih.gov/32966582/
https://pubmed.ncbi.nlm.nih.gov/33531712/
https://pubmed.ncbi.nlm.nih.gov/34643000/
https://pubmed.ncbi.nlm.nih.gov/32360126/
https://pubmed.ncbi.nlm.nih.gov/34156871/
https://pubmed.ncbi.nlm.nih.gov/33259812/
It is already known that SARS-CoV-2 triggers inflammatory responses and cell death in lung epithelial cells via the activation of caspase-8.
https://pubmed.ncbi.nlm.nih.gov/33037188/
The study team also observed that the S protein promoted caspase 3 expression, which suggests that caspase 3 is also involved in the induction of cell damage.
The spike protein also triggered apoptosis of intestinal cells by increasing caspase-3 and decreasing Bcl-2 according to the news study findings, which may underlie S protein promotion of cell damage.
Numerous recent studies also showed that cell death triggered by SARS-CoV-2 was associated with the induction of apoptosis via several mechanisms, such as caspase-8 and inflammasome activation and autophagy promotion.
https://pubmed.ncbi.nlm.nih.gov/33231615/
https://pubmed.ncbi.nlm.nih.gov/34461258/
https://pubmed.ncbi.nlm.nih.gov/33278357/
Such findings suggest that the SARS-CoV-2-induced inflammatory response is an important factor in cell damage, and TNF-α, interferon-γ (IFN-γ), reactive oxygen species (ROS), and phosphatidylinositol 3-hydroxykinase (PI3K)/threonine kinase (AKT)/mechanistic target of rapamycin (mTOR) signalling are involved in these mechanisms.
It is already known that the entry of the virus into target cells caused a cytokine “storm”, which led to severe organ or tissue injury, including lung injury.
Hence the study team used three cell lines derived from different tissues, HSIMECs, HCoEpiCs and hTIIAECs, and demonstrated that S protein induced the dysregulation of several inflammatory cytokines in these small intestine, colon and lung cells, including IL-6 and TNF-α.
Interestingly however, the S protein decreased the level of IL-10 instead of IL-13 in HSIMECs, but reduced the level of IL-13 instead of IL-10 in HCoEpiCs, which was consistent with hTIIAECs.
The study findings suggest that the changes in IL-10 and IL-13 induced by the S protein are associated with the cell type. S protein reduced TGF-β1 but not NF-κB levels in the supernatant of hTIIAECs, but this phenomenon was not observed in HSIMECs or HCoEpiCs.
The study data suggests that TGF-β1 is related to S protein-induced biological effects in lung cells. Extensive studies reported that cell damage induced by the S protein was related to TGF-β1 and NF-κB signalling in inflammation.
The study team further observed that treatment with an inhibitor of the TGF-β1, but not NF-κB, signalling pathway relieved the S protein-induced reduction in intestinal cell viability, which was promoted by the IKK/NF-κB pathway inhibitor BAY11-7082.
Such findings suggest that the TGF-β pathway contributes to intestinal cell damage via other pathways or mechanisms, and the NF-κB pathway does not play a significant role in this process.
Furthermore, inhibition of the TGF-β pathway, but not the NF-kB pathway, also relieved the spike protein-induced apoptotic injury in hTIIAECs and improved inflammatory cytokines, which suggest that the spike protein-activated TGF-β pathway, but not NF-kB p65, is involved in the process of lung cell damage.
The study findings are consistent with other past studies.
https://pubmed.ncbi.nlm.nih.gov/32505821/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7305506/
Significantly, a reduced concentration of TGF-β1 was found in the supernatant of hTIIAECs, which was inconsistent with the results of p-Smad2 and p-Smad3 in WB.
The study team proposed that the spike protein, or other factors, deplete extracellular TGF-β1, and the intracellular Smad pathway is activated by other mechanisms.
In another past study, it was observed that SARS-CoV-2 in severe COVID-19 induced a TGF-β-dominated chronic immune response that did not target itself, which is consistent with the results of multilevel proteomics analysis showing that the TGF-β pathway was specifically dysregulated by SARS-CoV-2.
https://pubmed.ncbi.nlm.nih.gov/33785765/
A subsequent in vitro experiment by the study team further demonstrated that the S protein triggered a transcriptional response related to activation of TGF-β signalling, which is required for S protein-mediated barrier dysfunction, via the induction of host factor proteins, such as angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and furin.
The study team concluded, “The S protein of SARS-CoV-2 triggered intestinal and lung epithelial cell injury and disorder of inflammatory factor secretion in vitro. Although the NF-κB and TGF-β pathways may not be involved in S protein induction of cell damage, blockade of the TGF-β, but not the NF-κB, pathway alleviated S protein-induced cell damage via other unknown mechanisms., Further research is needed to investigate these mechanisms.”
For the latest SARS-CoV-2 Research, keep on logging to Thailand
Medical News.
