Coronavirus Research: Singapore Study Shows That Mast Cell Activation Plays Key Role In Severe COVID-19 And Chymase Could Be An Important Biomarker
Source: Coronavirus Research Jun 04, 2021 4 years, 8 months, 2 weeks, 4 days, 3 minutes ago
Coronavirus Research: A new study by Singapore researchers from the Duke-NUS Medical School, Singapore General Hospital and the National University of Singapore along with support from scientists from the Duke University Medical Center at North Carolina-USA, has found that mast cells are major culprits in the etiology of severe and critical COVID-19.
 A Mast Cell. Image Credit: Sakurra / Shutterstock
A Mast Cell. Image Credit: Sakurra / Shutterstock
The study findings could lead to suitable therapeutic and preventive interventions to reduce the morbidity and mortality of this viral illness.
In their research abstract, the study team said that “Lung inflammation is a hallmark of COVID-19 in severely ill patients and the pathophysiology of disease is thought to be immune-mediated. Mast cells (MCs) are polyfunctional immune cells present in the airways, where they respond to certain viruses and allergens, often promoting inflammation. The study observed widespread degranulation of Mast Cells during acute and unresolved airway inflammation in SARS-CoV-2-infected mice and non-human primates. In humans, transcriptional changes in patients requiring oxygen supplementation also implicated cells with a Mast Cell phenotype. Mast Cell activation in humans was confirmed, through detection of the Mast Cell-specific protease, chymase, levels of which were significantly correlated with disease severity. These results support the association of Mast Cell activation with severe COVID-19, suggesting potential strategies for intervention.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.medrxiv.org/content/10.1101/2021.05.31.21255594v1
To date, the significant mortality rate associated with the ongoing COVID-19 pandemic has been linked to acute respiratory distress syndrome (ARDS) and multi-organ dysfunction. Mechanisms underlying the phenomenon remain unclear.
Mast cells are a type of white blood cell that is found in connective tissues all through the body, especially under the skin, near blood vessels and lymph vessels, in nerves, and in the lungs and intestines. They are also found in mucous membranes.
Typically mast cells are granulocytes, white blood cells with granules containing a number of potent chemicals such as histamine, serotonin, and protein-digesting enzymes such as chymase and tryptase. They also contain cytokines and lipid mediators, common to all granulocytes.
These mast cells have a long lifespan.
In adult life, they are found in mature form only within the tissues, while in the blood, only their immature precursors are found, forming less than 0.005% of the total blood cells. Tissue-resident mast cells express multiple pathogen recognition receptors on the surface and within the cytoplasm.
It has been found that mast cell degranulation produces inflammation, recruiting other immune cells such as monocytes and neutrophils, and T lymphocytes.
These mast cells also affect the vascular tone and permeability through the vasoactive contents of their granules. By so doing, they may induce tissue hypoxia via shunts, bypassin
g the capillary bed. This may result in damage to both the tissue and the vascular structure.
These cells have diverse phenotypes depending on the tissue of residence. For example, those in the lung of individuals with atopy express Immunoglobulin E (IgE) receptor, FcεR1 at higher levels relative to the skin, and are implicated in the severe inflammation of the lung that occurs in asthma.
While mast cells bring together immune responses against viruses and other pathogens, they may be harmful to the host as well, as shown by the above observations.
In the case if influenza, mast cell stabilization led to better control of lung lesions.
Significantly when mast cell activation remains at a high level systemically, the vascular and coagulation systems are affected. This is mediated by chymase and other granule enzymes. This is seen, for instance, in dengue hemorrhagic fever, with leaking blood vessels and clotting defects.
This new study adds to the already known knowledge by showing that mast cell degranulation is widespread in the lung tissue in both acute and recovering mice and non-human primate (NHP) animal models infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and in human COVID-19 patients.
Interestingly in animal models involving mice, mast cell degranulation was found in the airways, while resting mast cells were found in the healthy trachea. Tracheal inflammation and swelling were also found in other infected animals.
Importantly mouse chymase levels were high, this being a biomarker of mast cell activation, beginning to decrease with the reduction of the viral burden.
It was also found that genes relating to mast cell precursors found in peripheral blood were highly expressed in patients with severe COVID-19. This was accompanied by the upregulation of many host response pathways for the main mast cell products.
It was also found that Chymase from mast cells was present at high levels in the sera of these patients, further proof of mast cell activation in COVID-19. Thus, SARS-CoV-2 causes mast cell activation in vivo.
In non-human primates (NHPs), severe lung disease was observed in all animals, with areas of bleeding and fluid build-up. Necrotic patches were present on the lungs in half the cases. Degranulated mast cells were seen in many places within lung tissue, as well as free granules outside the mast cells.
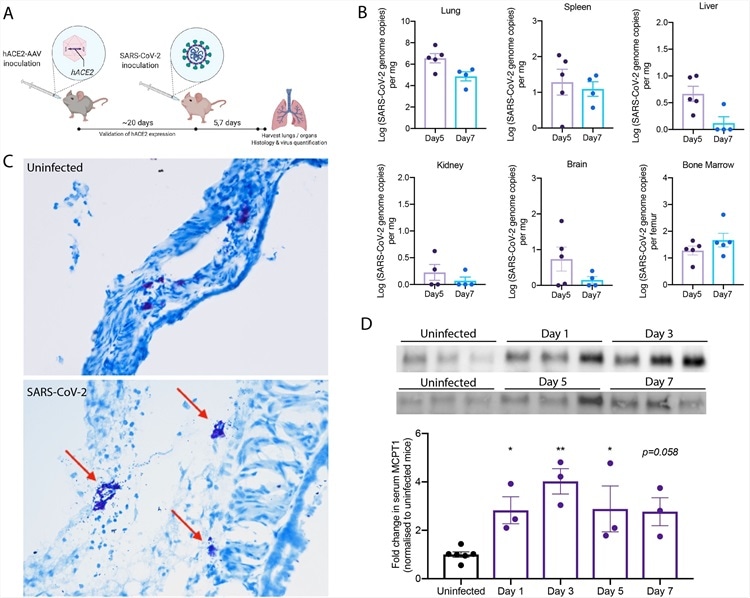 Degranulation of MCs in SARS-CoV-2 infected mice. (A) C57BL/6 mice were inoculated intranasally with hACE2-AAV to induce hACE2 expression in the airways. SARS-CoV-2 (2x107 TCID50) TCID-50) was inoculated intranasally into hACE2-AAV C57BL/6 mice. Blood was taken on days 1, 3, 5, and 7, and organs were harvested after 5 or 7 days for histology and virus quantification. (B) Virus quantification from the organs harvested shows detection in the lung, spleen, liver, kidney, brain, and bone marrow both Days 5 and 7. (C) Histology images of toluidine blue-stained trachea sections from uninfected and SARS-CoV-2 infected mice. Degranulating MCs (red arrow) could be observed in SARS-CoV-2 infected mice as well as tissue edema and airway narrowing. (D) Western blot images after chymase detection in serum Days 3, 5 and 7 post-infection shows systemic elevation of chymase, which was quantitated by densitometry from 3 individual mouse samples and presented as fold-increase over uninfected controls. Error bars represent the SEM. Chymase was significantly elevated in serum of infected mice compared to uninfected controls, determined by 1-way ANOVA with Dunnett’s post-test; *p<0.05, **p<0.01. Nonsignificant p-values below p=0.01 are shown on the graph.
Degranulation of MCs in SARS-CoV-2 infected mice. (A) C57BL/6 mice were inoculated intranasally with hACE2-AAV to induce hACE2 expression in the airways. SARS-CoV-2 (2x107 TCID50) TCID-50) was inoculated intranasally into hACE2-AAV C57BL/6 mice. Blood was taken on days 1, 3, 5, and 7, and organs were harvested after 5 or 7 days for histology and virus quantification. (B) Virus quantification from the organs harvested shows detection in the lung, spleen, liver, kidney, brain, and bone marrow both Days 5 and 7. (C) Histology images of toluidine blue-stained trachea sections from uninfected and SARS-CoV-2 infected mice. Degranulating MCs (red arrow) could be observed in SARS-CoV-2 infected mice as well as tissue edema and airway narrowing. (D) Western blot images after chymase detection in serum Days 3, 5 and 7 post-infection shows systemic elevation of chymase, which was quantitated by densitometry from 3 individual mouse samples and presented as fold-increase over uninfected controls. Error bars represent the SEM. Chymase was significantly elevated in serum of infected mice compared to uninfected controls, determined by 1-way ANOVA with Dunnett’s post-test; *p<0.05, **p<0.01. Nonsignificant p-values below p=0.01 are shown on the graph.
Also important, the greatest concentration of mast cells was seen within the areas of hemorrhage. These findings indicate that SARS-CoV-2 infection causes lung lesions with persistent mast cell activation at 21 days after acute infection.
As mature mast cells are not found in blood, their transcriptional profile was explored to detect systemic elevation of mast cell products. This showed that mast cell-associated gene expression increased with severe disease, some in the acute phase and some when the illness was resolving.
However these changes were not seen to evolve over time in mild COVID-19, though individual gene changes did occur. Overall, severe COVID-19 results in the activation of key immune receptors on mast cells, as shown by disrupted downstream pathways. Mast cells are therefore likely to be important in causing severe disease.
It was found that in human patients with severe COVID-19, mast cell maturation markers were increased.
Epithelial junction signaling was activated in response to mast cell proteases to increase vascular permeability. Similar was the case with pro-inflammatory pathways that are responsive to mast cell products.
The renin-angiotensin pathway was also perturbed, perhaps due to the activity of mast cell chymase which is responsible for angiotensin II production outside of ACE (angiotensin-converting enzyme) activity. In fact, chymase levels in hospitalized COVID-19 patients were markedly higher than in those with mild disease.
Significantly, chymase-mediated angiotensin II is found in atherosclerosis of the aorta and in early lung vascular disease, and some evidence suggests its implication in lung injury in COVID-19. Angiotensin II is elevated, pushing up the angiotensin I/angiotensin II ratio in severe COVID-19.
Importantly the difference was most marked when intubated patients were considered vs. outpatients with COVID-19.
The former also showed elevated markers of endothelial activation, which is associated with severe COVID-19 - again, as expected following mast cell activation. Thus, both high chymase levels and mast cell activation are characteristic of severe COVID-19.
Hence it can be deduced that mast cell activation is likely to increase the intensity of inflammation, delaying recovery and enhancing tissue injury, as suggested by the findings in mouse models. The NHP experiments indicate persistent inflammatory damage even after the infection subsides because of the antibody response.
Also mast cells may be activated in the resolving phase of infection by autoantibodies since they have to activate FcγRs on their surface for antigen- IgG antibody immune complexes. This may partly account for long COVID-19 but must be established by more research.
The study team says that it is possible that mast cell precursors in the blood are recruited and are responsible for the increased transcription of pro-inflammatory chemicals observed in this study. This is more likely since elevated chymase transcription was not identified, it being a specific product of mature mast cells.
The team added that these precursors may migrate into the lung since the chemokine CXCR2 is also raised in patients with severe COVID-19. Tissue-resident mast cells have a sentinel function, detecting allergens and infectious pathogens and recruiting many immune cell types into the inflamed tissues.
Also the coagulation and vascular events in some severe COVID-19 patients are also explained by mast cell activation, as these cells line the blood vessels within tissues. Their degranulation affects the lung vascular bed, causing endothelial damage and disrupting pulmonary blood flow and oxygenation, resulting in tissue damage.
Importantly a similar phenomenon is seen with dengue, where the virus does not directly infect the lungs but activates endothelial cells, causing microvascular leakage and hemorrhage. This is enhanced by mast cells, which help clear the virus in early dengue, but paradoxically worsen disease severity in the later stages.
Chymase Inhibitors and drugs targeting Mast Cells and their products are promising as a therapeutic strategy to prevent severe clinical courses in dengue infection and may bear similar promise in preventing severe COVID-19, which warrants further evaluation.
For the latest
Coronavirus Research keep on logging to Thailand Medical news.

